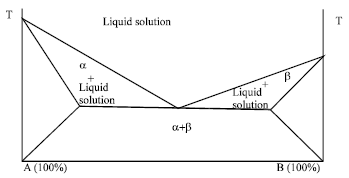
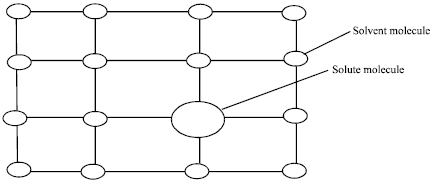
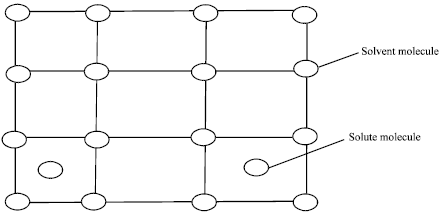
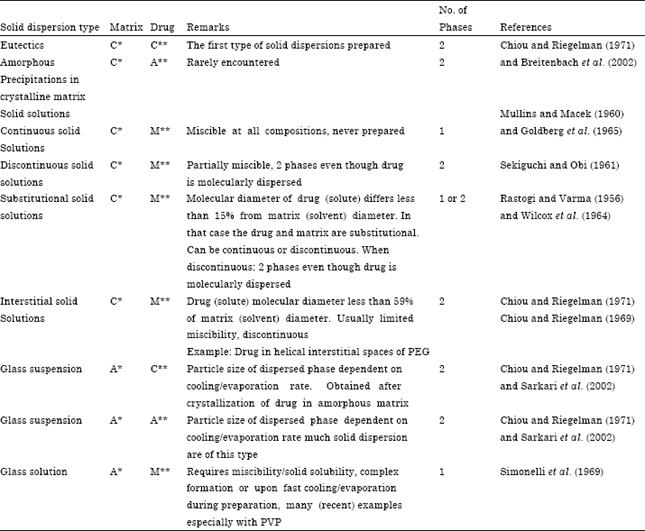
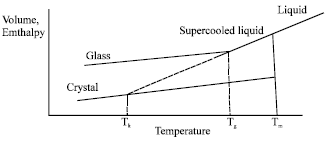
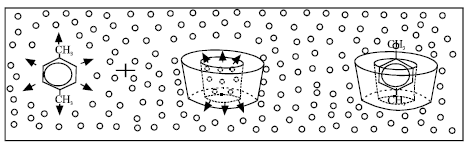
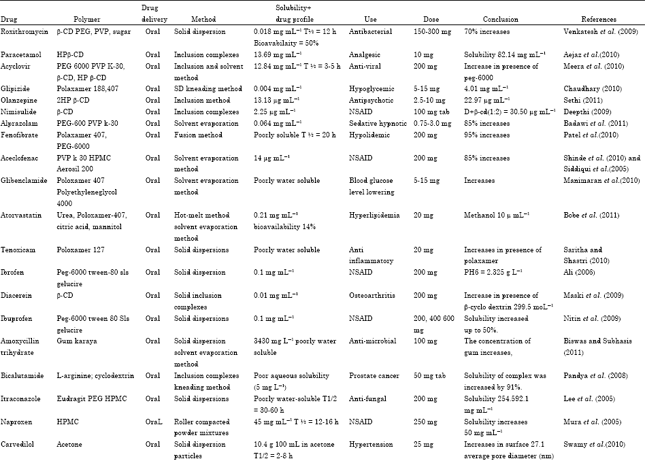
Review Article
An Exhaustive Review on Solubility Enhancement for Hydrophobic Compounds by Possible Applications of Novel Techniques
Rungta College of Pharmaceutical Sciences and Research, Kohka-Kurud Road, Bhilai, Chhattisgarh, India
Ajazuddin
Rungta College of Pharmaceutical Sciences and Research, Kohka-Kurud Road, Bhilai, Chhattisgarh, India
Tapan Kumar Giri
Rungta College of Pharmaceutical Sciences and Research, Kohka-Kurud Road, Bhilai, Chhattisgarh, India
Dulal Krishna Tripathi
Rungta College of Pharmaceutical Sciences and Research, Kohka-Kurud Road, Bhilai, Chhattisgarh, India
Vishal Jain
University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur, Chhattisgarh, India
Amit Alexander
Rungta College of Pharmaceutical Sciences and Research, Kohka-Kurud Road, Bhilai, Chhattisgarh, India