Research Article
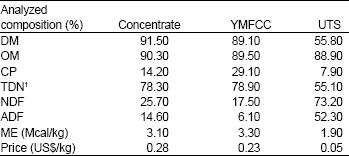
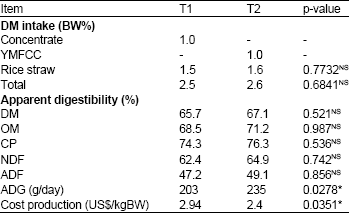
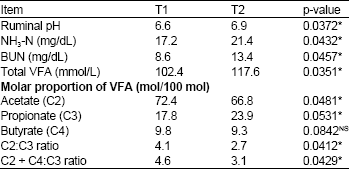
Effects of Supplementation of Yeast-Malate Fermented Cassava Chip as a Replacement Concentrate on Rumen Fermentation Efficiency and Digestibility of Nutrients in Cattle
Faculty of Agricultural Technology,
Pala Chaowarat
Faculty of Agricultural, Technology Rajabhat Maha Sarakham University, P 0 Box 44000, Thailand
Rungson Singhalert
Faculty of Humanities and Social Sciences, Rajabhat Maha Sarakham University, P 0 Box 44000, Thailand
Metha Wanapat
Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khan Kaen University, P.O. Box 40002, Thailand