Research Article
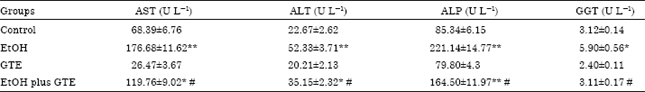
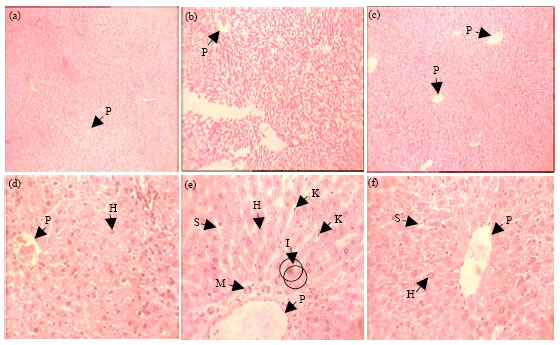
Aqueous Extract of Iranian Green Tea Prevents Lipid Peroxidation and Chronic Ethanol Liver Toxicity in Rat
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
H.R. Rahimi
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
A.R. Ebadollahi-Natanzi
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
B. Minaei-Zangi
Department of Anatomy, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
O. Sabzevari
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran