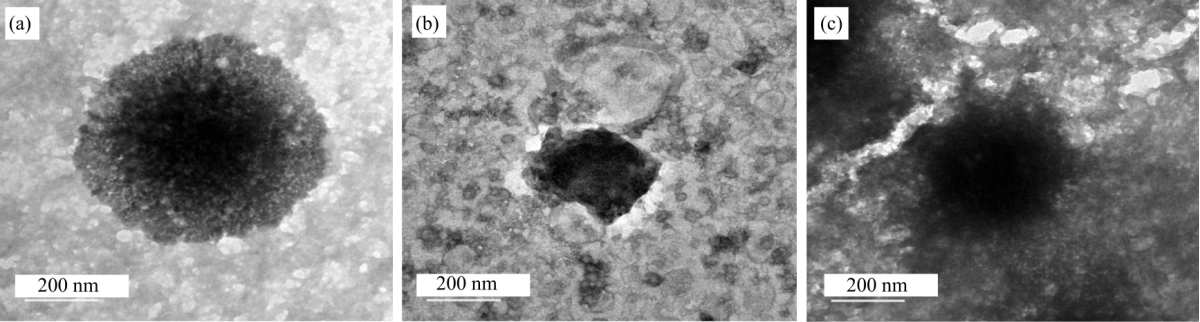
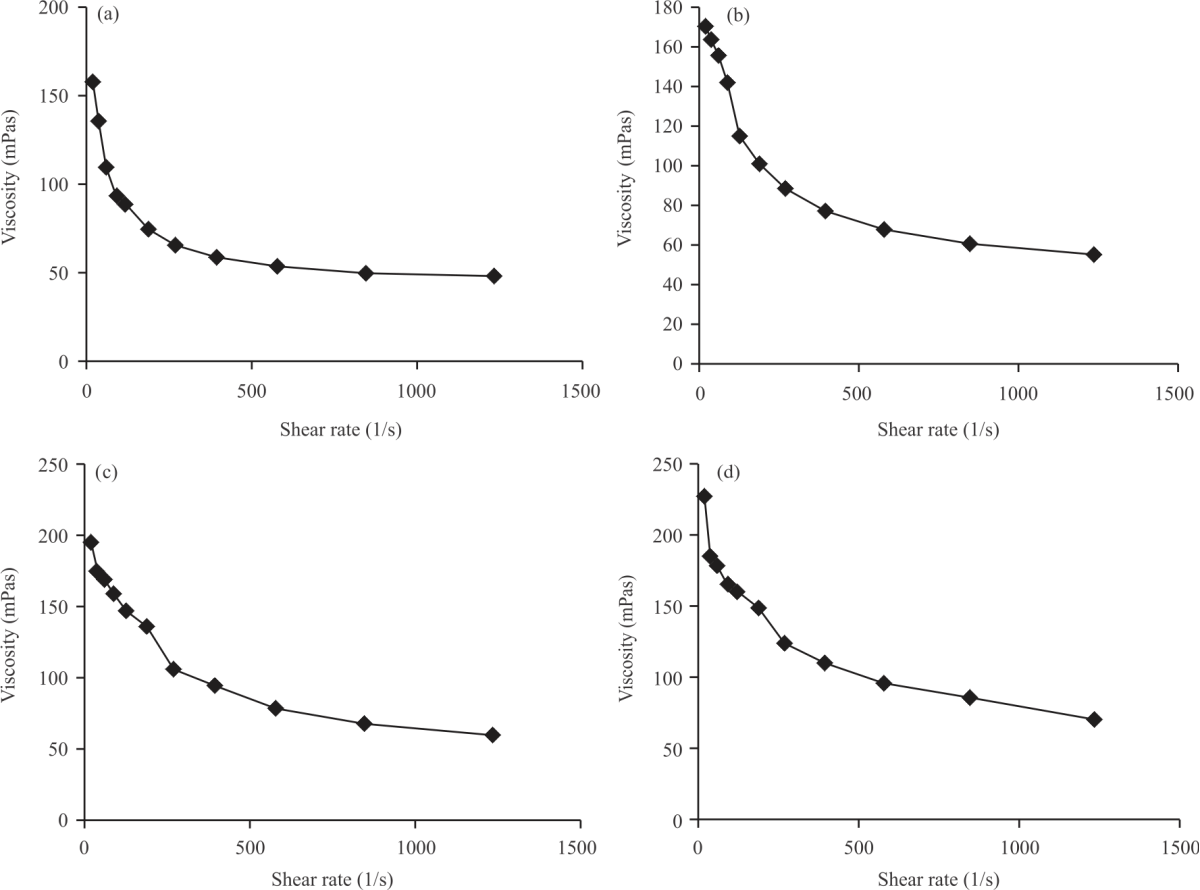
Research Article
Functional Flavoured Mandarin Fermented Goat Milk with Algae Oil Nanoemulsion
Department of Dairy Research and Technology, Food Technology Research Institute, Agricultural Research Centre (ARC), 9 El Gamma St., Giza, Egypt
Aly A. Shahin
Department of Dairy Research and Technology, Food Technology Research Institute, Agricultural Research Centre (ARC), 9 El Gamma St., Giza, Egypt
Mohamed F. Al-Okbay
Department of Fats and Oils Research, Food Technology Research Institute, Agricultural Research Centre (ARC), 9 El Gamma St., Giza, Egypt
Tarek N. Soliman
Department of Dairy, Food Industries and Nutrition Research Institute, National Research Centre El-Buhouth St., 12622 Dokki, Cairo, Egypt
LiveDNA: 20.23162