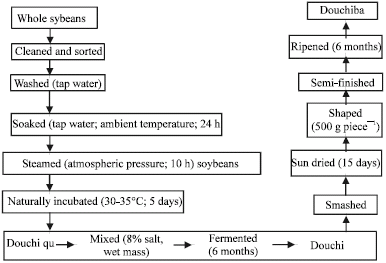
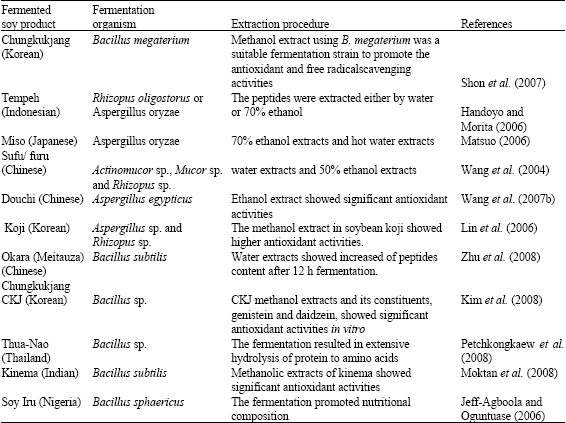
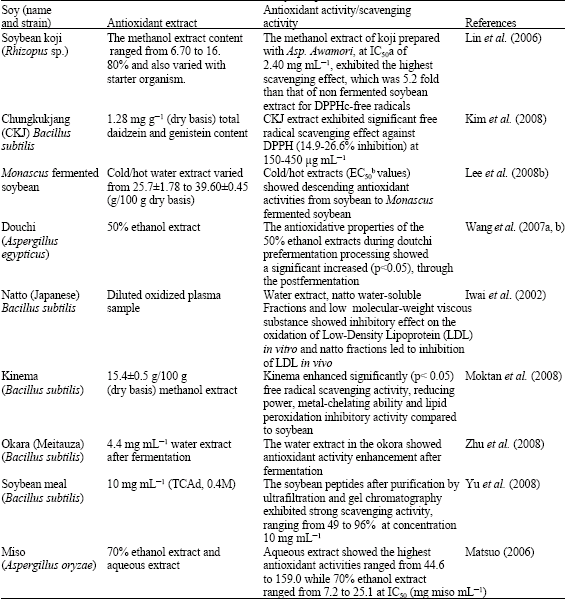
Research Article
Fermented Soybean Products: Some Methods, Antioxidants Compound Extraction and their Scavenging Activity
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800 Lihu Road, Wuxi, 214122, Jiangsu Province, Peoples Rupublic of China
S. Yong-Hui
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800 Lihu Road, Wuxi, 214122, Jiangsu Province, Peoples Rupublic of China
J. Sun
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800 Lihu Road, Wuxi, 214122, Jiangsu Province, Peoples Rupublic of China
L. Guo-Wei
State Key Laboratory of Food Science and Technology, Jiangnan University, No. 1800 Lihu Road, Wuxi, 214122, Jiangsu Province, Peoples Rupublic of China