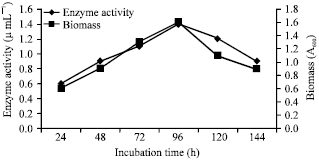
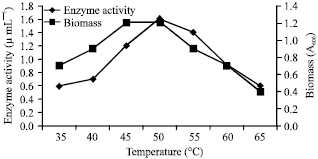
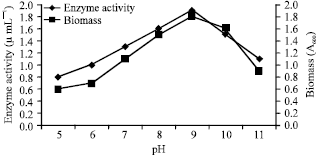
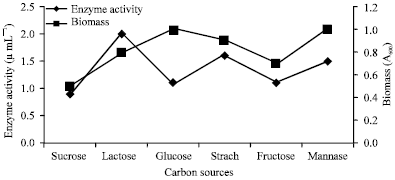
Research Article
Production, Optimization and Characterization of Fibrinolytic Enzyme by Bacillus subtilis RJAS19
Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai, Tamil Nadu, India
R. Rakshitha
Department of Biotechnology, Kalasalingam University, Srivilliputtur, Tamil Nadu, India
M. Annu Vidhya
Department of Biotechnology, Kalasalingam University, Srivilliputtur, Tamil Nadu, India
P. Sharon Jennifer
Department of Biotechnology, Kalasalingam University, Srivilliputtur, Tamil Nadu, India
Sandip Prasad
Department of Biotechnology, Kalasalingam University, Srivilliputtur, Tamil Nadu, India
M. Ravi Kumar
Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai, Tamil Nadu, India
P.T. Kalaichelvan
Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai, Tamil Nadu, India