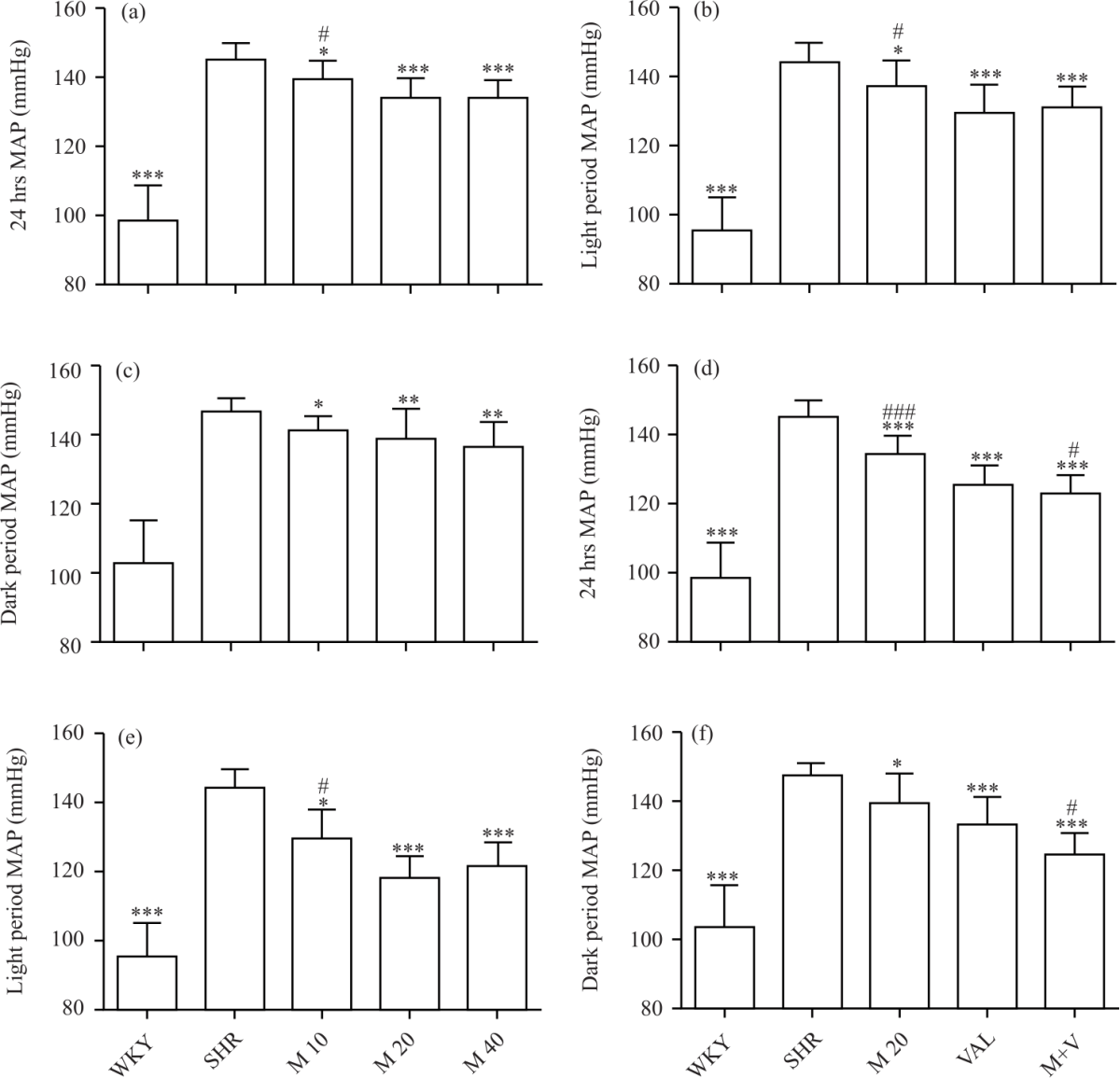
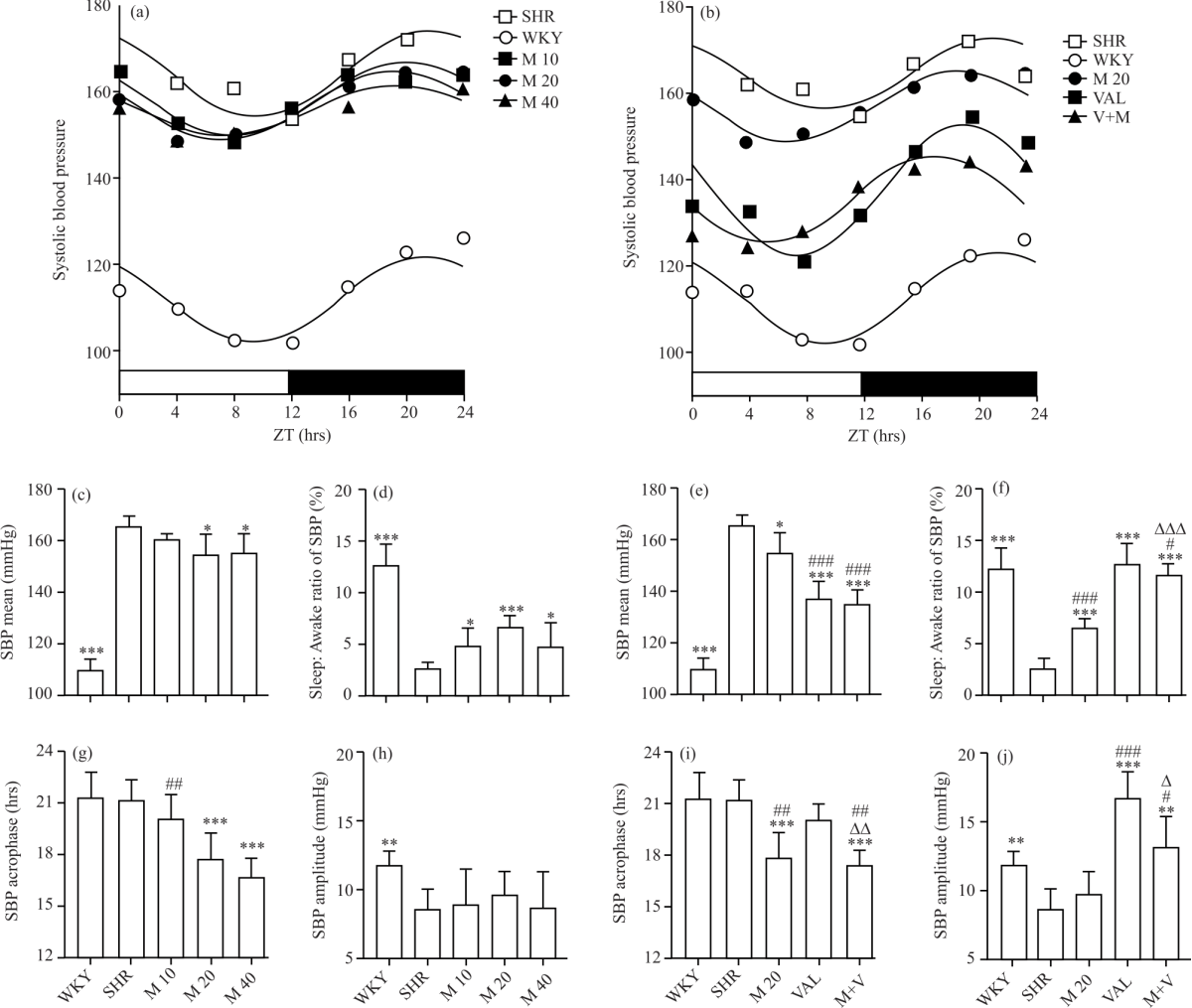
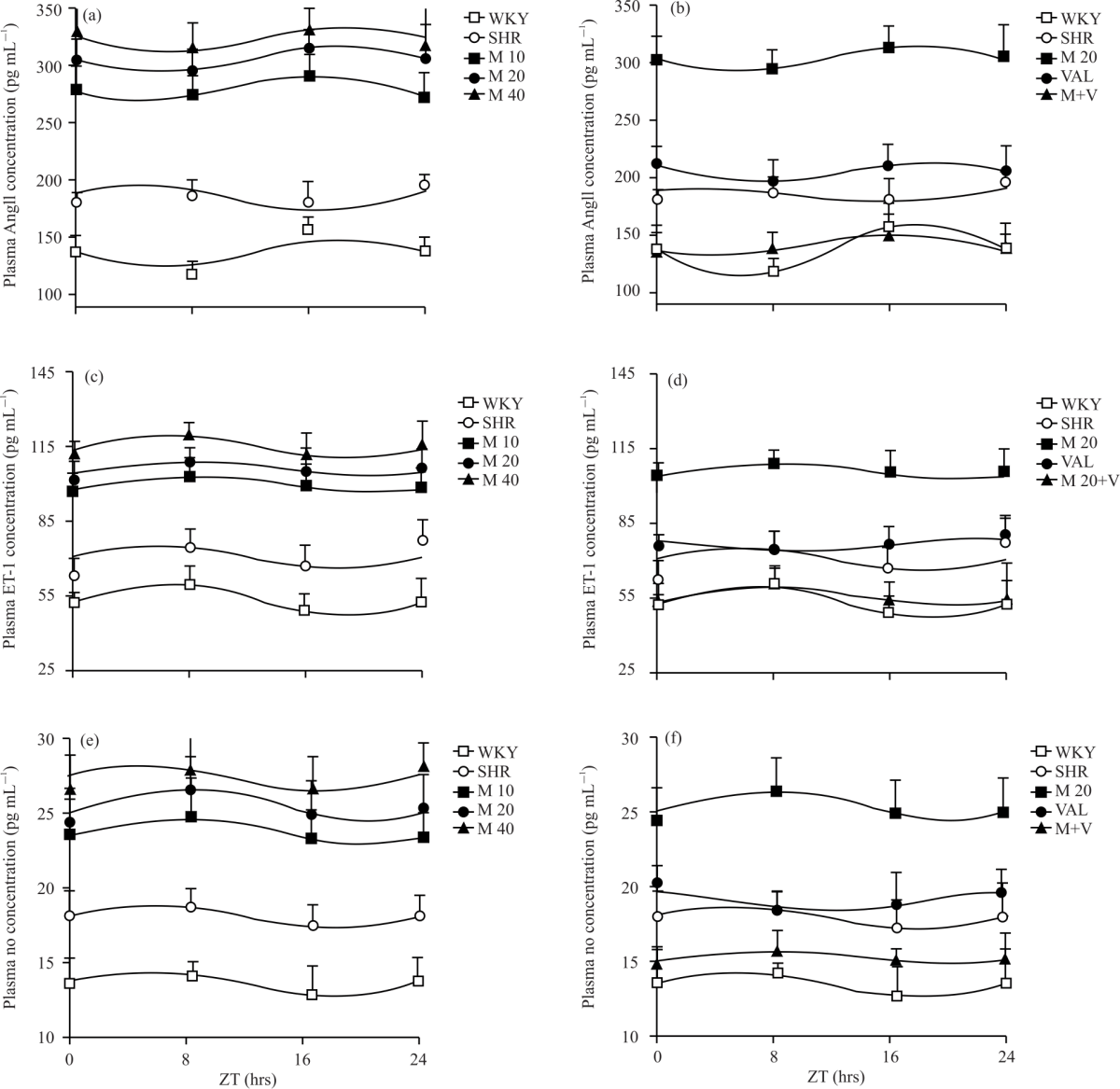
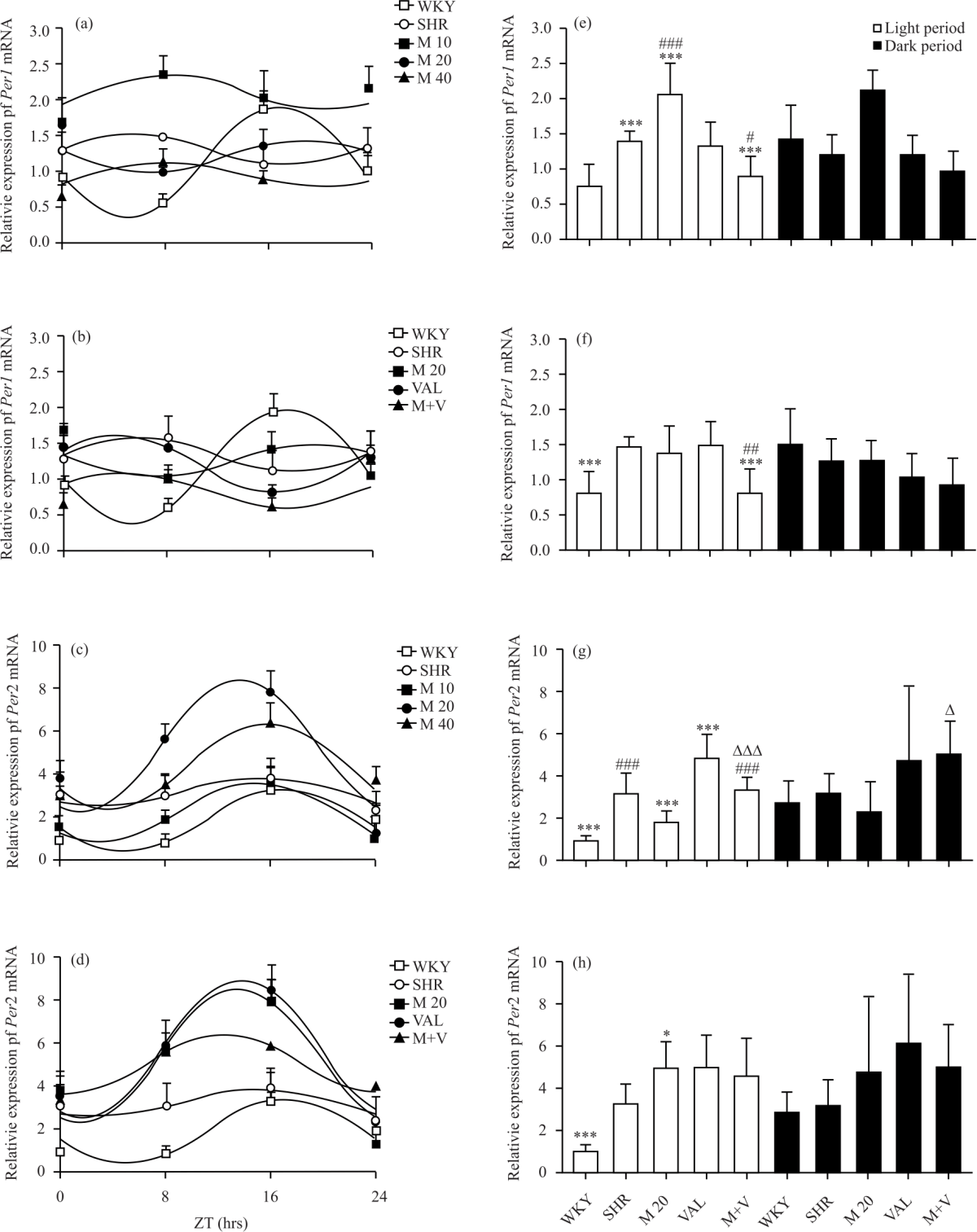
Research Article
Combination of Valsartan and Melatonin to Treat Non-Dipping Hypertension Rats via Circadian Clock System
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Ding Yanyun
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Jin Wan
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Wang Yaqin
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Chen Lu
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Zhang Wen
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Wang Wusan
School of Pharmacy, Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Ma Zhangqing
School of Pharmacy, Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
Luan Jiajie
Department of Pharmacy, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui 241001, People's Republic of China
LiveDNA: 86.35745