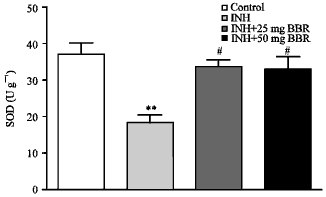
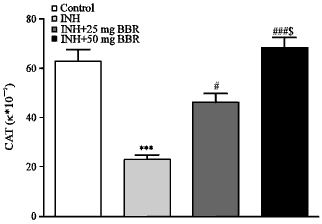
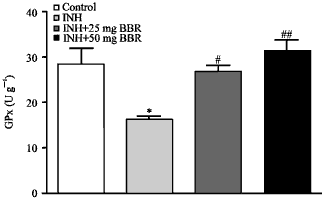
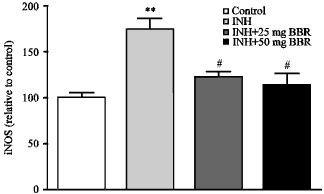
Research Article
Berberine Attenuates Isoniazid-Induced Hepatotoxicity by Modulating Peroxisome Proliferator-Activated Receptor γ, Oxidative Stress and Inflammation
Physiology Division, Department of Zoology, Faculty of Science, Beni-Suef University, Egypt
Mousa O. Germoush
Department of Biology, Faculty of Science, Aljouf University, Sakaka, Saudi Arabia
Ayman S. Soliman
Department of Physiology, Faculty of Medicine, Beni-Suef University, Egypt