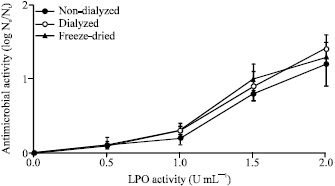
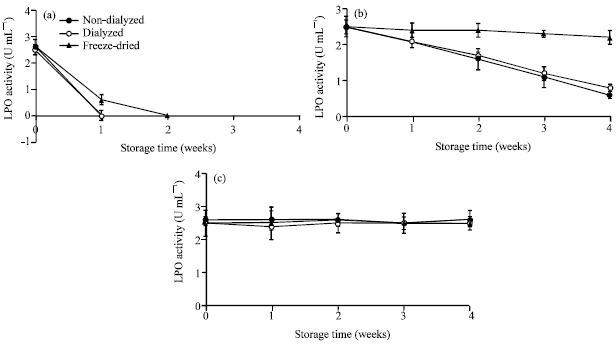
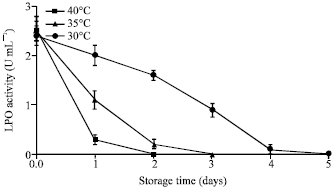
Research Article
Application of Lactoperoxidase System Using Bovine Whey and the Effect of Storage Condition on Lactoperoxidase Activity
Department of Applied Biological Sciences, Faculty of Agriculture, Kagawa University, 2393, Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0795, Japan
Masahiro Ogawa
Department of Applied Biological Sciences, Faculty of Agriculture, Kagawa University, 2393, Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0795, Japan
Shigeru Hayakawa
Department of Applied Biological Sciences, Faculty of Agriculture, Kagawa University, 2393, Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0795, Japan