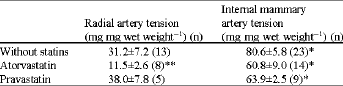Research Article
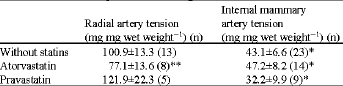
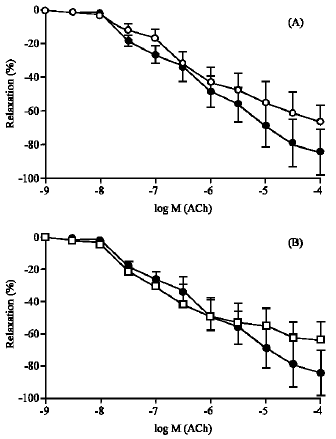
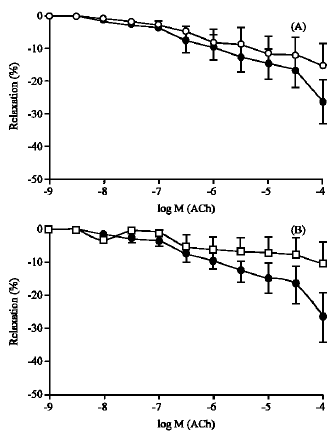
Lack of Effect of Atorvastatin or Pravastatin on the Endothelium-Dependent Relaxation in Segments of Human Vessels
Cardiovascular Department, Clinical Hospital, University of Chile, Independencia 1027, Santiago7, Chile
Gianni Pinardi
Pharmacology Program ICBM, Faculty of Medicine, University of Chile, Independencia 1027, Santiago7, Chile
Jaime Zamorano
Cardiovascular Department, Clinical Hospital, University of Chile, Independencia 1027, Santiago7, Chile
Ernesto Larrain
Cardiovascular Department, Clinical Hospital, University of Chile, Independencia 1027, Santiago7, Chile
Ramiro J. Zepeda
Pharmacology Program ICBM, Faculty of Medicine, University of Chile, Independencia 1027, Santiago7, Chile
Rodrigo Castillo
Cardiovascular Department, Clinical Hospital, University of Chile, Independencia 1027, Santiago7, Chile
Juan Espinoza
Cardiovascular Department, Clinical Hospital, University of Chile, Independencia 1027, Santiago7, Chile
Hugo F. Miranda
Pharmacology Program ICBM, Faculty of Medicine, University of Chile, Independencia 1027, Santiago7, Chile