Research Article
Possible Roles of the Leaves of Two Paspalum Species in Salinity Tolerance
Department of Botany and Microbiology, Faculty of Science, University of Lagos, Akoka-Yaba, Lagos, Lagos State, Nigeria
INTRODUCTION
In an earlier experiment, on the growth response of Paspalum vaginatum and P. scrobiculatum in relation to salinity over a short period of time, it was reported that both species significantly accumulated sodium and chloride ions in their leaves as external concentration increased (Shonubi and Okusanya, 2007). Paspalum vaginatum accumulated more sodium and chloride ions in its dead leaves than the fresh leaves, whereas the converse was the case in P. scrobiculatum. These findings indicated that both species may be employing sodium and chloride ions in their salinity response. It was also suggested that one way by which P. vaginatum tolerates salinity may be by a mechanism of moving excess salt from young leaves to older and possibly dying leaves. The young leaves of P. vaginatum as opposed to P. scrobiculatum also contained relatively high amount of water which may be used to dilute excess salt to tolerable level. Thus, the leaves of these two species appear to play some roles in their salinity tolerance.
In view of the above, an experiment was designed to determine the changes in the concentrations of sodium, chloride and related ions as well as water, chlorophyll and sugar contents in the young and old leaves of these Paspalum species over a long period of time. Since, the greatest and most significant difference in growth in the two species measured by dry weight occurred at 100 mM salinity (Shonubi and Okusanya, 2007), this salinity concentration was employed in this experiment.
Many different mechanisms have been proposed to explain salinity tolerance in halophytes (Brown, 1978; Yeo, 1998; Tester and Davenport, 2003; Mansour and Salama, 2004) and in non-halophytes (Greenway and Munns, 1980; Hasegava et al., 2000; Munns, 2002). While, the regulation of ion uptake in the plant is a commonly accepted mechanism and it has been shown to be employed in many species (Gorham et al., 1985; Matoh et al., 1988; Munns and James, 2003; Garthwaite et al., 2005) and in P. vaginatum (Shonubi and Okusanya, 2007), there is no record of the possible movement of salt from young leaves to the old dying leaves.
This study aimed to determine whether salt could be moved from the young leaves to the old leaves or whether the excess salt in the shoot is diverted to and accumulated in old dying leaves. There is limited evidence of extensive re-circulation of shoot Na+. This suggests that Na+ transport is highly unidirectional and results in progressive accumulation of Na+ as leaves age (Tester and Davenport, 2003). This experiment might also, help to determine the possible roles played by the leaves of these two Paspalum species in their salinity tolerance.
The experiment was carried out in October 2002. The plant cuttings from each species, the preparation of 50 and 100 mM sea water solutions and the experimental conditions were as described in the earlier experiment (Shonubi and Okusanya, 2007). The cuttings were allowed to stabilize and establish for 1 week watering daily with one-fifth strength Hoagland’s solution. Thereafter, they were watered with 50 mM seawater solution for 4 days and stepped up to 100 mM seawater solution. After 1 week the first harvest was made. Three other harvests were made at 10 days interval.
At the beginning of the experiment, the third leaf from the bottom was tagged so as to allow its subsequent identification at harvest and to follow the changes in ion concentration over the experimental period. Watering procedure, experimental conditions and analytical methods were as described earlier (Shonubi and Okusanya, 2007).
At each harvest, 10 plants were chosen at random. The youngest fully expanded leaf and the tagged third leaf from the bottom were first removed and sub-samples were analyzed for water, sugar and chlorophyll contents. The water and chlorophyll analysis was as reported earlier (Shonubi and Okusanya, 2007), while the sugar content was determined by anthrone method (Yemm and Willis, 1954).
The remaining plants had their roots cleaned off adhering soil particles and gently rinsed in distilled water. Both the tiller number and leaf number were determined in order to follow the leaf growth in both species. The separated young leaves, the tagged leaves and the remaining plants were separately dried to constant weight. The total dry weight was determined at each harvest and the dried young and tagged leaves were analyzed for their sodium, potassium, calcium, magnesium and chloride contents. Analysis of variance was used to compare the values between the harvests and between the species.
The third tagged leaf from the bottom in both species remained the third leaf from the bottom in most cases, but it became old and was dying with harvest. However, the young leaf harvested was positioned between 21 and 45 from the bottom in P. vaginatum and between 16 and 20 in P. scrobiculatum indicating much rapid growth in P. vaginatum.
| Table 1: | Changes in tiller number, leaf number and total dry weight of Paspalum vaginatum and P. scrobiculatum with harvest. Mean of 10 replicates (±SE) |
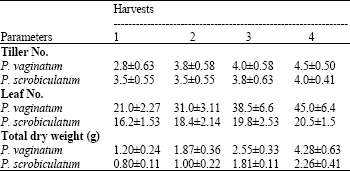 | |
Table 1 showed that there was a significant increase in growth as measured by dry weight in P. vaginatum with harvest (p<0.05%), but growth in P. scrobiculatum was not significantly different between the first two harvests. The dry weight in P. vaginatum was nearly double that in P. scrobiculatum at each harvest. Tiller and leaf number increased with harvest in both species but the increase was more remarkable in P. vaginatum.
Mineral ion concentration: In the young leaf of P. vaginatum, sodium ion was virtually the same at all harvests but the reverse was the case in P. scrobiculatum. The values in P. scrobiculatum were significantly higher (p<1%) than in P. vaginatum. In the tagged leaf, there was a significant increase in sodium content with harvest and the values were also significantly higher (p<5%) than in the young leaf in P. vaginatum. In P. scrobiculatum, there was significant increase in the sodium ion content values with harvest (p<5%) but the values were not significantly different from those in the young leaf (Table 2).
The values for chloride ions follow the same pattern as for sodium ion in both species. It is noteworthy that the chloride ion values in both young and tagged leaves in P. scrobiculatum were not significantly different, but the values in the tagged leaves of P. vaginatum were significantly higher (p<5%) than the values in P. scrobiculatum (Table 2).
As for potassium ion content, there was no significant difference with harvest in the young leaves of P. vaginatum, but with the tagged leaf, there was a progressive and significant decrease with harvest. In P. scrobiculatum, there was also no significant difference in the potassium ion concentration with harvest in both the young and tagged leaf. Values in the tagged leaves in both species were significantly lower ( p<5%) than in the young leaves (Table 2).
For calcium ion, there was an increase in value with harvest in both the young and tagged leaf in P. vaginatum. The values for the tagged leaf are significantly higher (p<5%) than in the young leaf. In P. scrobiculatum, there was an insignificant increase in calcium ion values with harvest in both the young and tagged leaves, but the values in the tagged leaf were significantly higher (p<5%) than in the young leaf. Overall, P. vaginatum accumulated significantly more calcium ion in tagged old leaf than P. scrobiculatum (Table 2).
| Table 2: | Changes in ion concentrations (Molar) of young and tagged leaves in Paspalum vaginatum and P. scrobiculatum with harvest |
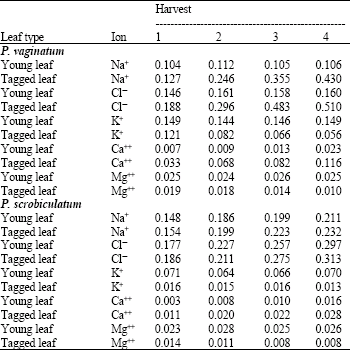 | |
The pattern of the result for magnesium ion content was the same in both species as there was no difference in the values with harvest in the young leaf but there was significant decrease (p<5%) with harvest in the tagged leaf, possibly due to the loss of chlorophyll (Table 2, 3).
Chlorophyll content: Table 3 showed that the chlorophyll content in the young leaf of P. vaginatum was virtually the same throughout the harvests. There was a slight increase in P. scrobiculatum with harvest. For the tagged old leaf in P. vaginatum, chlorophyll content was significantly lower than in the young leaf (p<5%) and it decreased with harvests until the last harvest when the old leaf became non-chlorophylous. In P. scrobiculatum, chlorophyll concentration in the tagged leaf was significantly lower (p<1%) than in the young leaf and it decreased rapidly with harvest to the extent that they were non-chlorophylous at the last two harvests.
Leaf water content: Leaf water content was virtually constant in the young leaf of P. vaginatum at all harvest but the values decreased slightly with harvest in P. scrobiculatum.
| Table 3: | Changes in chlorophyll, water and sugar contents in young and tagged leaves of Paspalum vaginatum and P. scrobiculatum with harvest |
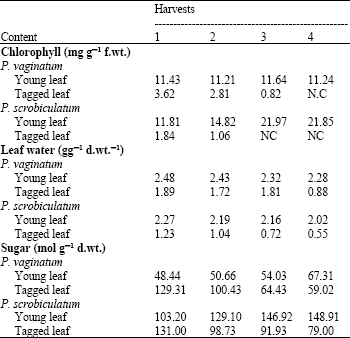 | |
NC: Non chlorophyllous | |
The tagged leaf in P. vaginatum had significantly lower leaf water content than the young leaf only in the last harvest. In P. scrobiculatum, leaf water content in the tagged leaves significantly decreased with harvest (p<5%) and their values were also significantly lower than in P. vaginatum (Table 3).
Sugar concentration: There was a slight but insignificant increase in sugar concentration with harvest in the young leaf of P. vaginatum, but in the tagged leaf, there was a significant decrease (p<1%) most likely due to the loss of chlorophyll at the last two harvests (Table 3). In P. scrobiculatum, the pattern was the same as in P. vaginatum, but the values in the young leaf were significantly higher than in P. vaginatum (p<5%).
The uptake of water and nutrients by the roots of plants is important to the survival of plants. The uptake of nutrients probably depends on the need of the plant species and their availability in the growth medium. In saline environment, there is continuous uptake of salt and many species employ different mechanisms to cope with life in this environment. The mechanism of salt tolerance in halophytes and non-halophytes has been extensively dealt with by Greenway and Munns (1980), Gorham et al. (1985), Cheeseman (1988), Hasegawa et al. (2000) and Yokoi et al. (2002). In halophytes, the mechanisms include an interplay of physiological processes such as the restriction of salt uptake by the roots, the compartmentalization of salt in the tonoplast, excretion of excess salt through glands, the storage of salt in non-essential parts, the accumulation of water (increase succulence) to dilute accumulated salt and increased sugar concentration in the leaf (Flowers et al., 1977; Cheeseman, 1988; Blum, 1994; Hasegawa et al., 2000; Yokoi et al., 2002).
In this experiment, the increased salt concentration in the tagged old leaf with harvest as compared to the near constant salt concentration in the young leaf of P. vaginatum (Table 2) indicates that excess salt was being stored in the old leaves. Since, the tagged leaf became old and non chlorophylous as the plant grew, it means that the plant may be regulating the amount of salt in the plant by storing excess salt in the old dying leaves (Karley et al., 2000; Zhang and Bumwald, 2001; Tester and Davenport, 2003; Shonubi and Okusanya, 2007). As the excess salt became toxic to the old leaves, the destruction of the chlorophyll set in leading to the death of the tagged old leaf. However, it is not certain whether the excess salt was from the young leaves or from uptake from the roots.
Evidence from this experiment showed that P. scrobiculatum is unable to regulate the uptake of salt and its transfer to the young leaves by the significantly high salt concentration in the young and tagged leaves as compared to those in P. vaginatum (Table 2). This has been reported for other non-halophytes (Matsushita and Matoh, 1991; Schachtman and Munns, 1992; Muhling and Lauchli, 2002). The species also appear unable to redistribute the sodium and chloride ions between the young and old leaves, because of the similar values in both the young and tagged leaves (Table 2).
The decreased water content in the tagged leaf in both species indicates that leaf water accumulation may not be employed by these species in salinity tolerance. While, Shonubi and Okusanya (2007) reported no significant decrease in water content as salinity increased in P. vaginatum in a short time experiment, the result in a long time experiment showed otherwise. This could mean a switch in mechanism of salt tolerance with time.
Sugar accumulation in leaves is a possible method used by plants in salinity tolerance by increasing osmotic potential (Zhu, 2002). This investigation indicates that P. scrobiculatum may be employing this mechanism to cope with salinity stress even at 10% salinity in view of the significantly higher values of sugar in the young leaf than in its tagged leaf (Table 3). Paspalum vaginatum does not appear to be using this mechanism possibly because it is not under salinity stress as it has extremely good growth at this salinity.
Increased calcium content in plants in saline medium is reported to serve as ameliorating agent (Bassett, 1980; Okusanya and Ungar, 1984; Shonubi and Okusanya, 2007). The significantly higher calcium values in the tagged leaf in both species (Table 2) indicate that accumulation of calcium by the leaf may be another way by which the species moderately tolerate salinity effects.
It is concluded that the leaves of the two Paspalum species play some important roles, albeit different roles in their salinity tolerance. While in P. vaginatum, the old leaves store excess salt thereby keeping the salt in young leaves within tolerable range and also accumulating water and calcium ion to ameliorate salt stress, in P. scrobiculatum, the young leaves seem to accumulate sugar while the old leaf accumulates calcium ion to ameliorate salt stress.
Author thank the authority of the University of Lagos for permission to carry out this project. Author is also very grateful to Prof. O.T Okusanya for his review and comments on the manuscript. Many thanks to Mrs. Sue Scanzillo for her help.