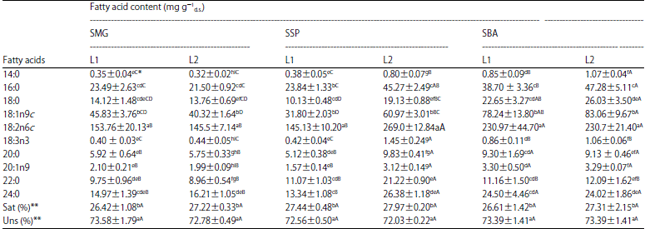
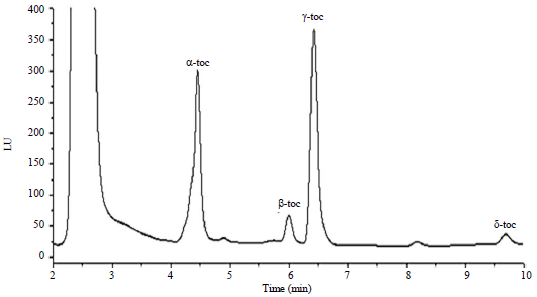
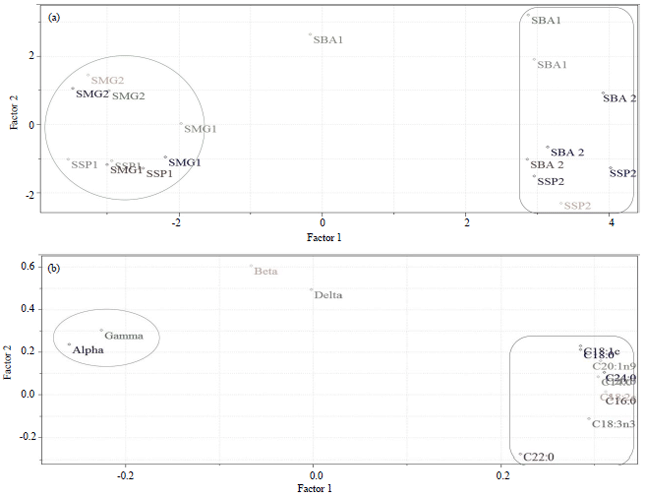
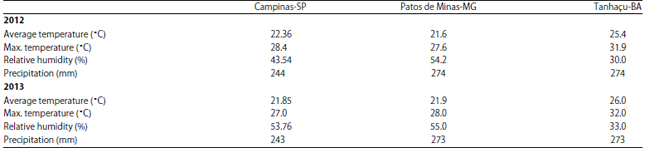
Research Article
Quantitative Profile of Fatty Acids and Tocopherols in Tamarind Seeds (Tamarindus indica L.) From Different States of Brazil
Nucleus of Graduation in Agroindustry, Federal University of Sergipe (UFS), Nossa Senhora da Gl�ria, SE, Brazil
LiveDNA: 55.20386
Cristiano Augusto Ballus
Department of Food Science and Technology, Federal University of Santa Maria (UFSM), Santa Maria, RS, Brazil
Wellington da Silva Oliveira
Department of Food Science, Faculty of Food Engineering, State University of Campinas (UNICAMP), Campinas, SP, Brazil
Jose Teixeira Filho
School of Agricultural Engineering, State University of Campinas (UNICAMP), Campinas, SP, Brazil
Helena Teixeira Godoy
Department of Food Science, Faculty of Food Engineering, State University of Campinas (UNICAMP), Campinas, SP, Brazil