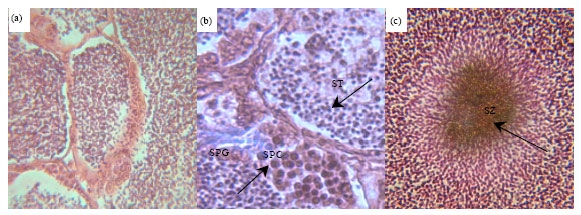
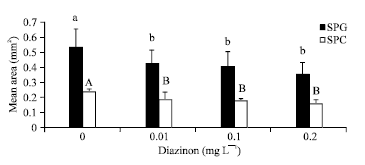
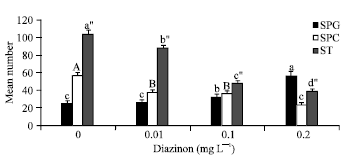
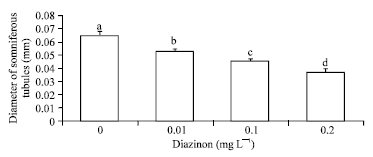
Research Article
In vitro Effects of Diazinon on Male Reproductive Tissue and Sperm Motility of Caspian Kutum (Rutilus frisii kutum)
Department of Fisheries Science, Faculty of Natural Resource, University of Tehran, Karaj, Iran
B. Mojazi Amiri
Department of Fisheries Science, Faculty of Natural Resource, University of Tehran, Karaj, Iran
A.R. Mirvaghefi
Department of Fisheries Science, Faculty of Natural Resource, University of Tehran, Karaj, Iran
M.A. Nemtollahi
Department of Fisheries Science, Faculty of Natural Resource, University of Tehran, Karaj, Iran