Research Article
Antifungal Activity of Plant Products for the Management of Fruit Rot Infection in Chillies
Department of Plant Pathology, Faculty of Agriculture, Annamalai University, Annamalai Nagar, Tamil Nadu, 608 002, India
S. Usharani
Department of Plant Pathology, Faculty of Agriculture, Annamalai University, Annamalai Nagar, Tamil Nadu, 608 002, India
Ancy P. George
Department of Plant Pathology, Faculty of Agriculture, Annamalai University, Annamalai Nagar, Tamil Nadu, 608 002, India









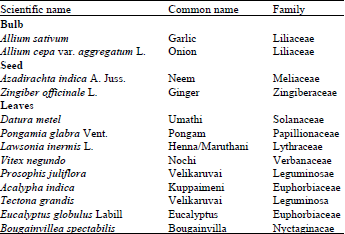
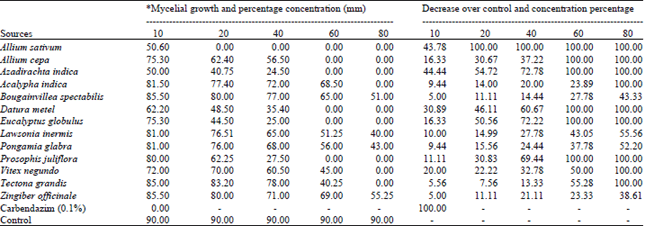
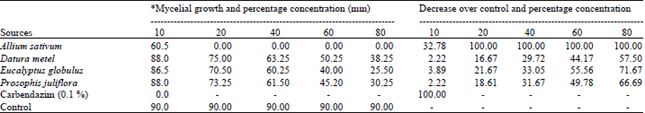
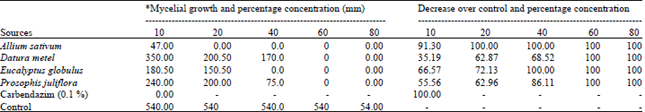
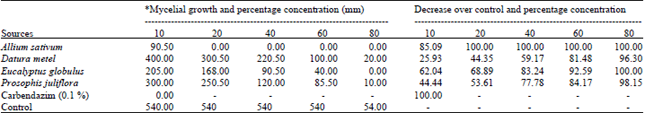
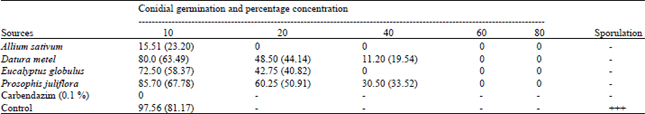
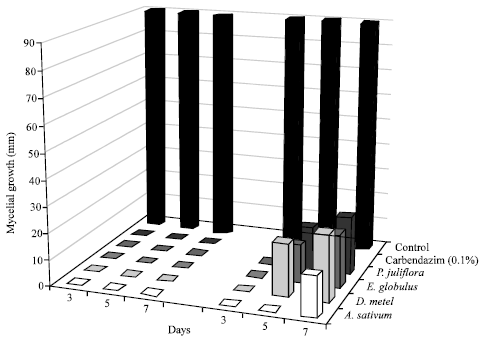
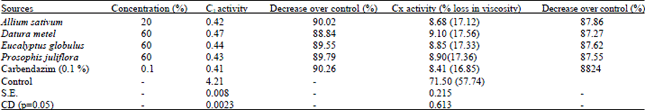
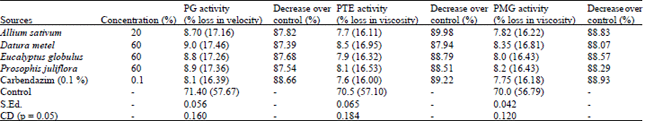
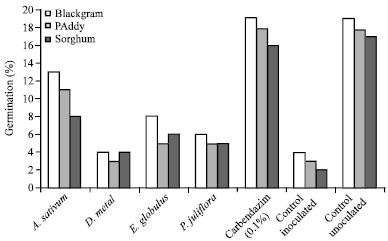
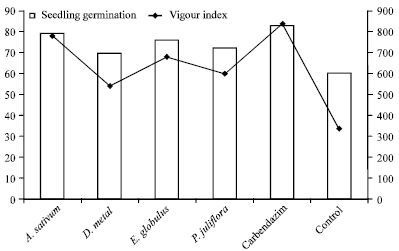
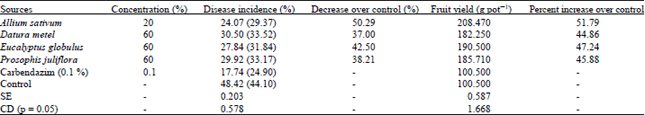
shamal salveen raj Reply
dear authors this paper was very useful.