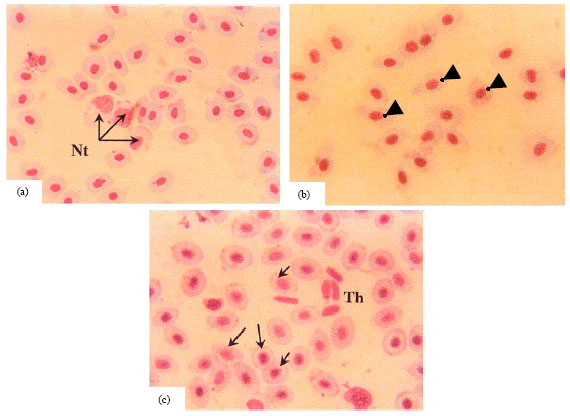
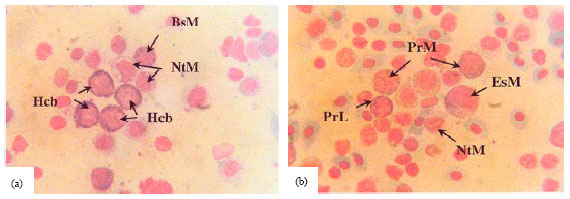
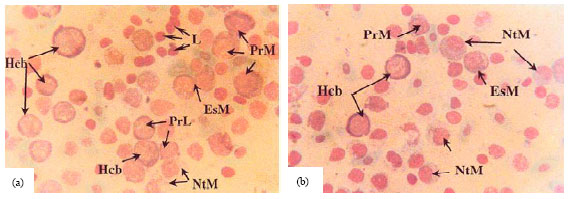
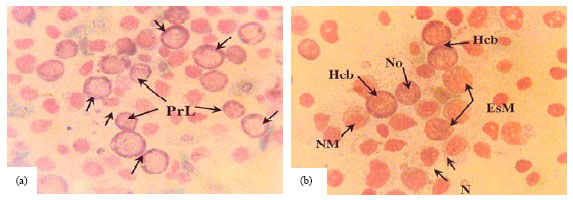
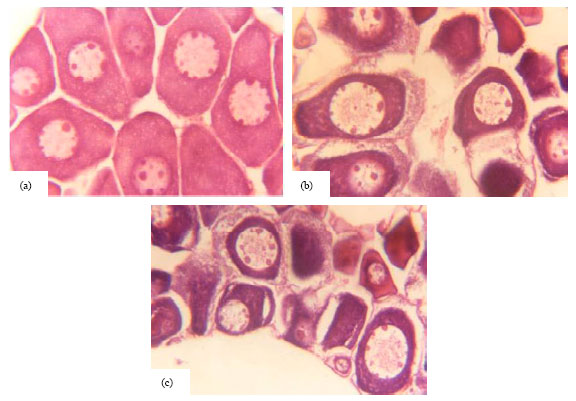
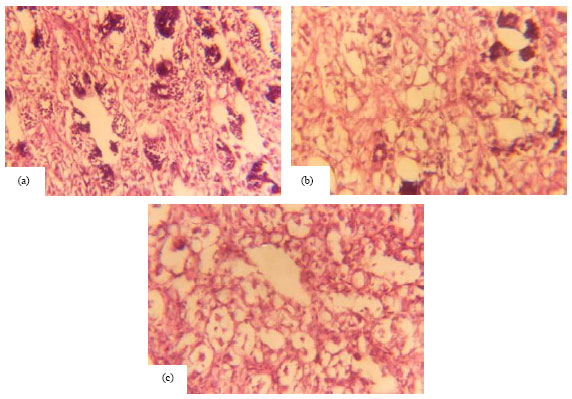
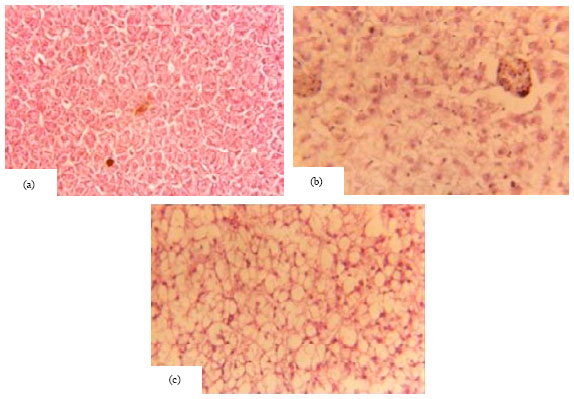
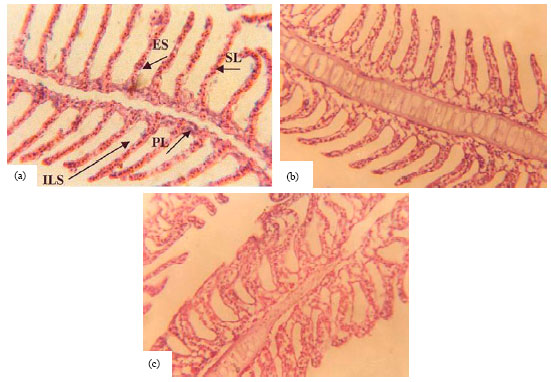
Research Article
Effect of Polluted Water from the Egyptian Eastern Mediterranean Coast on Reproductive, Toxicological and Hematological Characteristics of Siganus rivulatus
National Institute of Oceanography and Fisheries, Kaiet Bay, Alexandria, Egypt
O.M. Wahbi
National Institute of Oceanography and Fisheries, Kaiet Bay, Alexandria, Egypt
Z.A. El-Greisy
National Institute of Oceanography and Fisheries, Kaiet Bay, Alexandria, Egypt