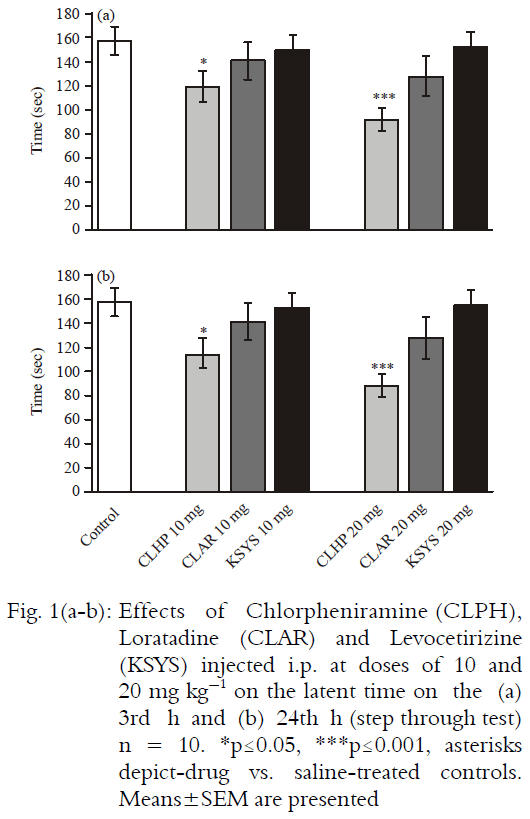
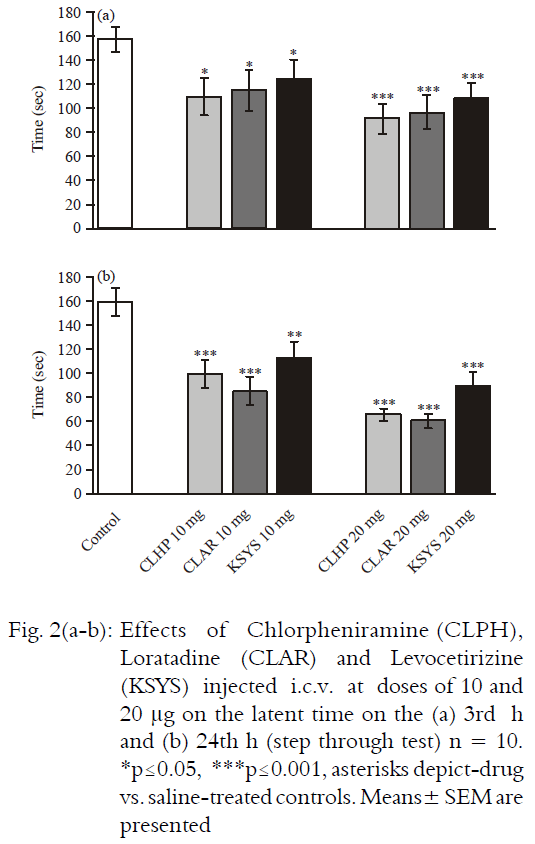
Research Article
Comparative Study of Learning and Memory Effects of Antihistamines Applied by Different Routes in Rats
Department of Behavior Neurobiology, Institute of Neurobiology, Bulgarian Academy of Sciences, Acad. G. Bonchev Str., Bl. 23, 1113 Sofia, Bulgaria
Roman Tashev
Department of Pathophysiology, Medical University of Sofia, 1 Zdrave Str., 1431 Sofia, Bulgaria
Stiliana Belcheva
Faculty of Pre-School and Primary School Education, SU "Sv. Kl. Ohridsky", 69A Shipchenski Prohod St., 1574 Sofia, Bulgaria