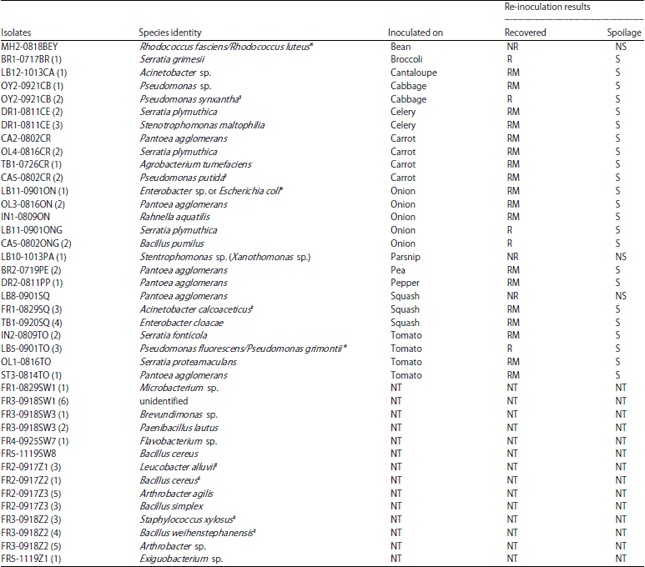
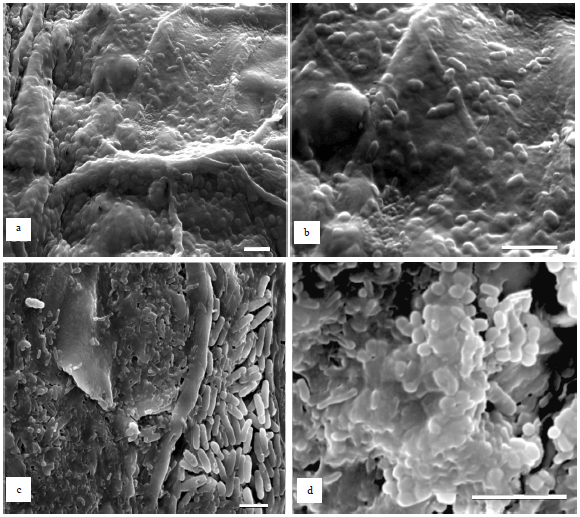
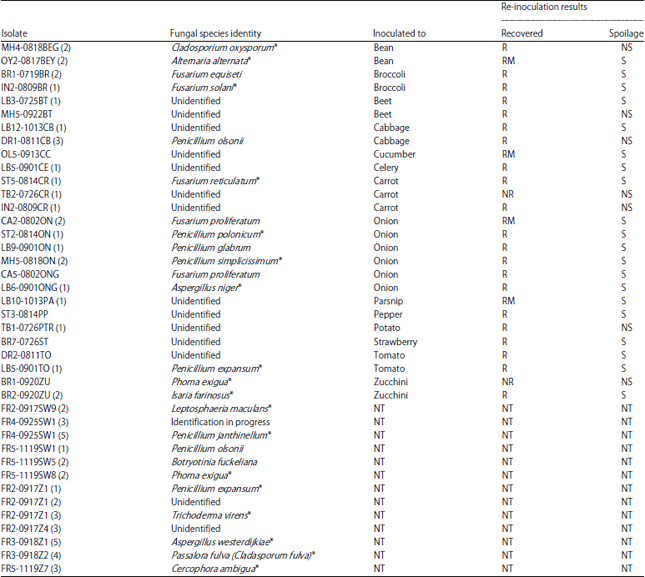
Research Article
Characterization of Microorganisms from Fresh Produce in Alberta, Canada Reveals Novel Food-spoilage Fungi
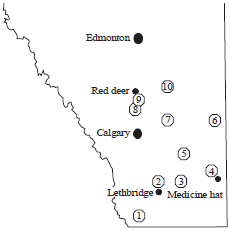
Crop Diversification Centre South, Alberta Agriculture and Rural Development, 301 Horticultural Station Road East, Brooks, AB, T1R 1E6, Canada
N. Butler
Innovotech Inc. 101-2011 94 Street, Edmonton, AB, T6N 1H1, Canada
W. Dmytriw
Alberta Innovates Technology Futures, P.O. Box 4000, Vegreville, AB, T9C 1T4, Canadaz
S. Rajput
Alberta Innovates Technology Futures, P.O. Box 4000, Vegreville, AB, T9C 1T4, Canadaz
LiveDNA: 91.10748
D.A. Burke
Crop Diversification Centre South, Alberta Agriculture and Rural Development, 301 Horticultural Station Road East, Brooks, AB, T1R 1E6, Canada
R.J. Howard
Crop Diversification Centre South, Alberta Agriculture and Rural Development, 301 Horticultural Station Road East, Brooks, AB, T1R 1E6, Canada RJH Ag Research Solutions Ltd., P.O. Box 1456, Brooks, AB, T1R 1C3, Canada