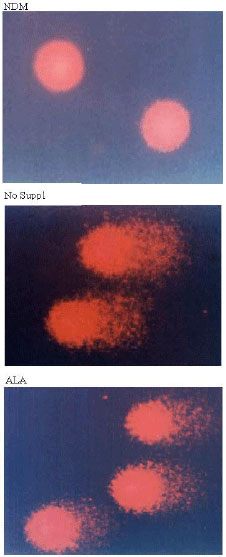
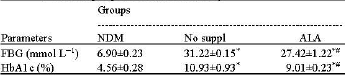
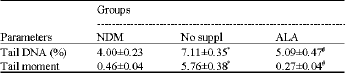
Research Article
Oxidative Stress and DNA Damage in Streptozotocin-Induced Diabetic Rats: Effects of Alpha Lipoic Acid
Biomedical Department, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur, 50300, Malaysia
R. Nor Fadilah
Department of Forensic Science, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia
A. Ezlan
Biomedical Department, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur, 50300, Malaysia
O. Khairul
Department of Forensic Science, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia
A.B. Mokhtar
Department of Pathology, Faculty of Medicines, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia
M. Jamaludin
Biomedical Department, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur, 50300, Malaysia