Research Article
Epoxidation of Vegetable Oils and Fatty Acids: Catalysts, Methods and Advantages
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Jumat Salimon
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia









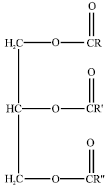
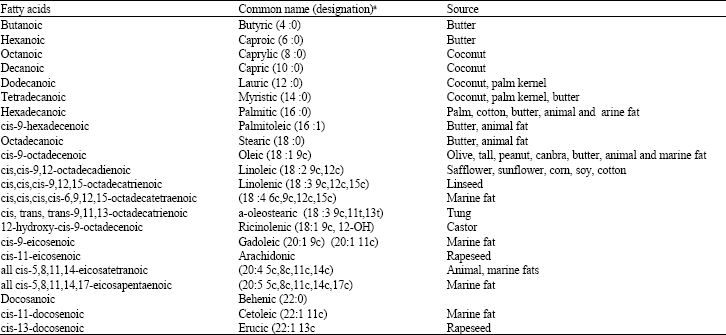
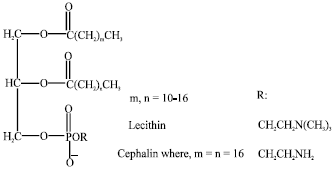
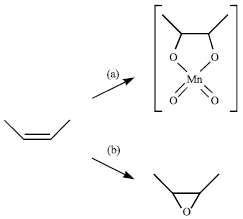
Abraha Reply
I need this paper