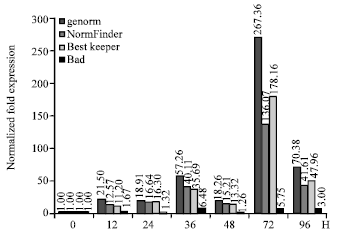
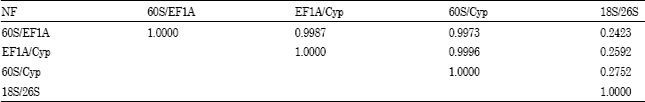
Research Article
Identification and Validation of Internal Control Genes for Gene Expression in Wheat Leaves Infected by Strip Rust
Triticeae Research Institute, Sichuan Agricultural University, Wenjiang, Sichuan, 611130, China
Ya-Xi Liu
Triticeae Research Institute, Sichuan Agricultural University, Wenjiang, Sichuan, 611130, China
Helene Rocheleau
Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, 960 Carling Ave, Ottawa, On, K1A 0C6, Canada
Therese Ouellet
Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, 960 Carling Ave, Ottawa, On, K1A 0C6, Canada
Guo-Yue Chen
Triticeae Research Institute, Sichuan Agricultural University, Wenjiang, Sichuan, 611130, China