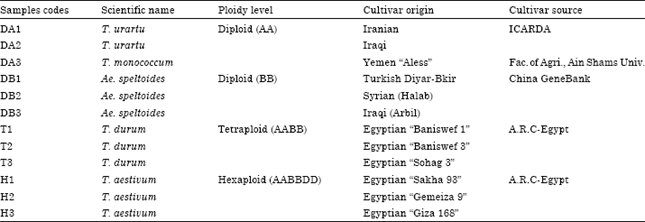
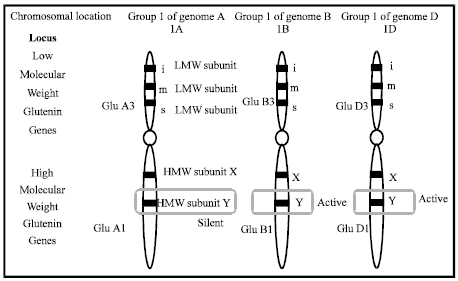
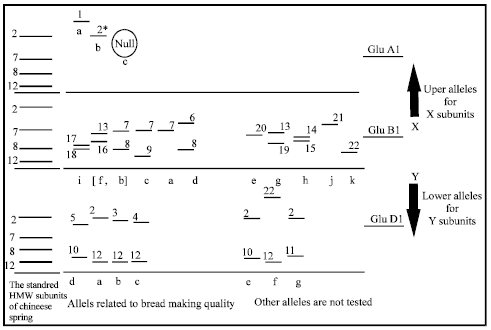
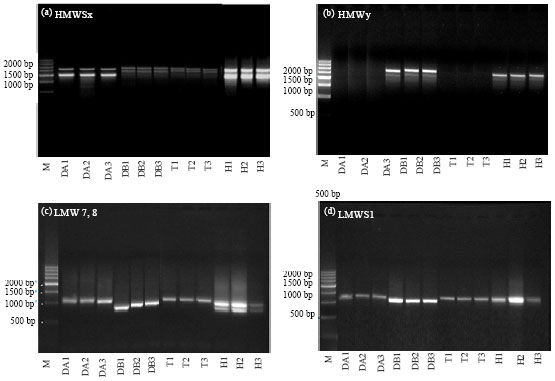
Research Article
Discriminative Features of Some High and Low Molecular Weight Glutenin Genes in Different Wheat Species
Department of Genetics and Cytology, Molecular Cytogenetics Group, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, Giza, Egypt
E.A.A. Abd-Elhady
Department of Genetics and Cytology, Molecular Cytogenetics Group, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, Giza, Egypt
S.A. Edris
Department of Genetics, Faculty of Agriculture, Ain Shams University, Egypt
F.M. El Domyati
Department of Genetics, Faculty of Agriculture, Ain Shams University, Egypt
N.R. Abdelhamid
Department of Genetics and Cytology, Molecular Cytogenetics Group, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, Giza, Egypt
A.H.M. Hassan
Department of Genetics and Cytology, Molecular Cytogenetics Group, Genetic Engineering and Biotechnology Division, National Research Center, Dokki, Giza, Egypt
A.A. El Seoudy
Department of Genetics, Faculty of Agriculture, Ain Shams University, Egypt