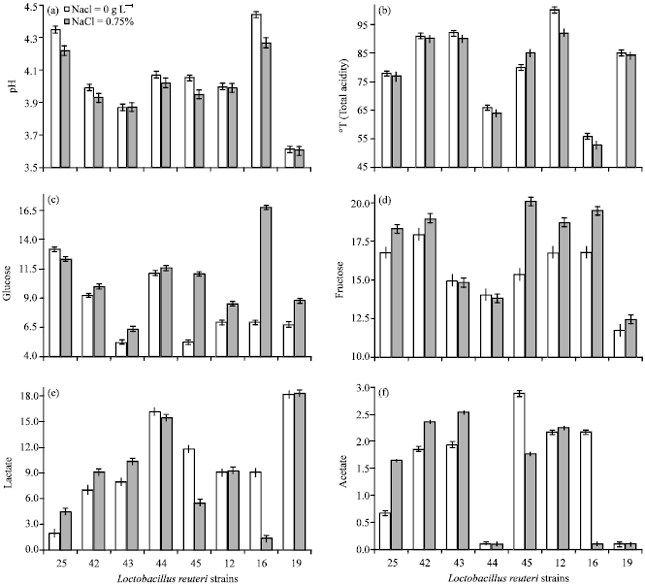
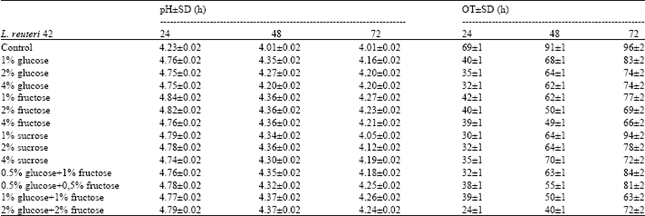
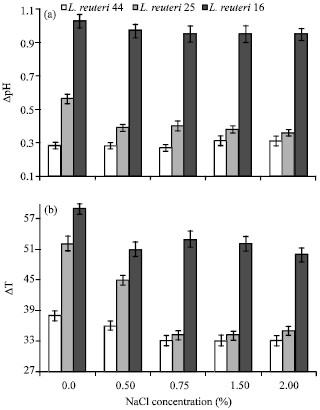
Research Article
Evaluation of Lactobacillus reuteri Strains for Pumpkin (Cucurbita pepo L.) Juice Fermentation
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
I. Denina
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
A. Fomina
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
L. Sakirova
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
L. Auzina
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
A. Patetko
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia
D. Upite
Institute of Microbiology and Biotechnology, Laboratory of Industrial Microbiology and Food Biotechnology, University of Latvia, 4 Kronvalda blvd, LV-1010, Riga, Latvia