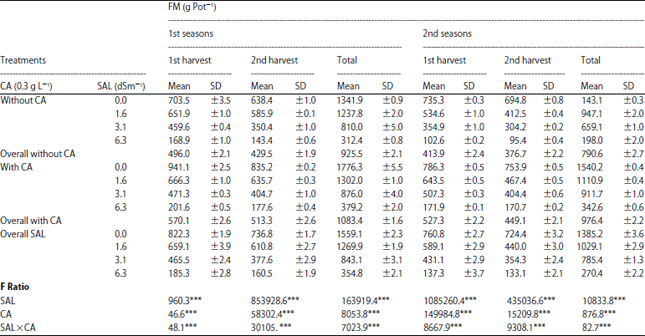
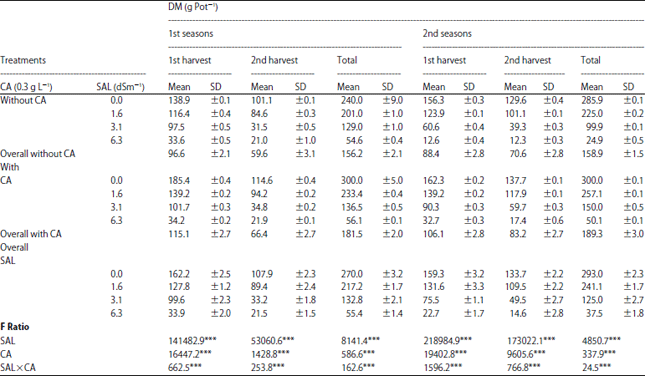
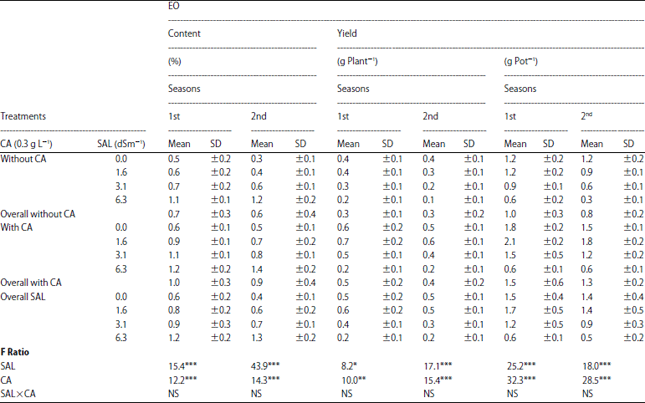
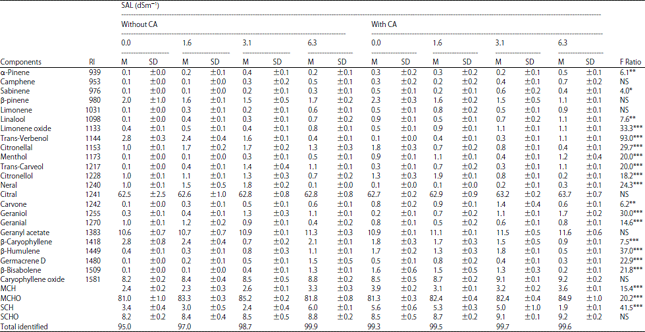
Research Article
Citric Acid Affects Melissa officinalis L. Essential Oil Under Saline Soil
Department of Botany, National Research Centre, 12311 Dokki, Cairo, Egypt
LiveDNA: 20.13590
Iman Mahmoud Talaat
Department of Botany, National Research Centre, 12311 Dokki, Cairo, Egypt
Khalid Ali Khalid
Department of Medicinal and Aromatic Plants, National Research Centre, 12311 Dokki, Cairo, Egypt
LiveDNA: 20.3160