Research Article
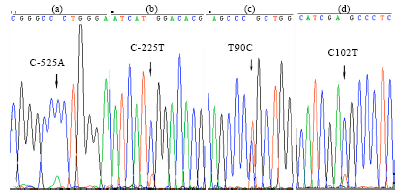
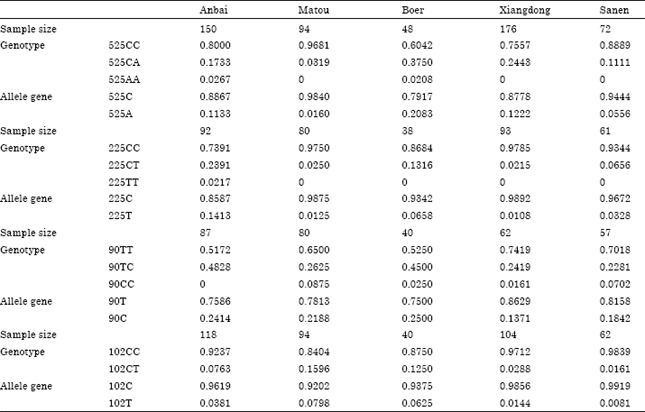
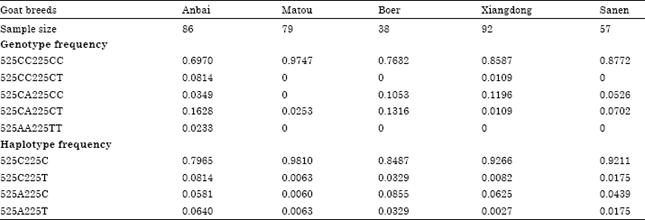
The Polymorphism in 5 'Regulatory Region and Exon 13 of PRKAG3 Gene and its Distribution Pattern in Different Goat Breeds
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Hong-Quan Chen
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Jie Qin
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Yin-Jian Zhu
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Hua Chen
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Gong-Wei Chen
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Ya-Nan Xie
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Zhong-Ting Pan
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Ming-Hui Jiao
School of Animal Science and Technology, Anhui Agricultural University, Hefei, 230036, The People`s Republic of China
Sheng-Qiang Huang
School of Animal Science and Technology, Hunan Agricultural University, Changsha, 410128, The People`s Republic of China
Ming-Xing Chu
Key Laboratory of Farm Animal Genetic Resources and Utilization of Ministry of Agriculture, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193, The People`s Republic of China