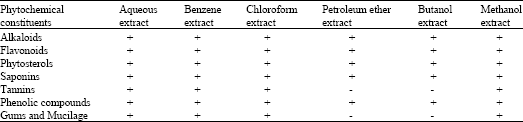
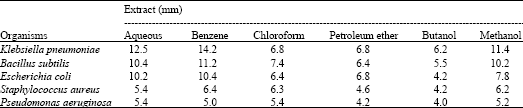
Research Article
Screening of Secondary Metabolites and Antibacterial Activity of Acacia concinna
Department of Biotechnology, Gogate Joglekar College,Ratnagiri-415612 University of Mumbai, Maharashtra, India
V.V. Chavan
Department of Zoology, Gogate Joglekar College,Ratnagiri-415612 University of Mumbai, Maharashtra, India
A.S. Kulkarni
Department of Zoology, Gogate Joglekar College,Ratnagiri-415612 University of Mumbai, Maharashtra, India