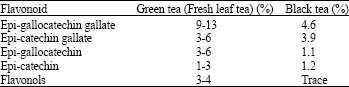
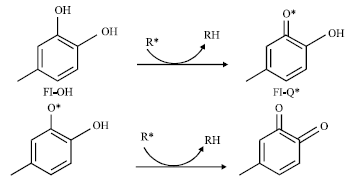
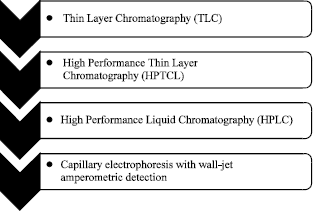
Research Article
Recent Updates on Free Radicals Scavenging Flavonoids: An Overview
A.S.B.A.S.J.S.M. College of Pharmacy, Bela (Ropar), Punjab, India
Rachna Kumria
M.M. College of Pharmacy, M.M. University, Mullana (Ambala), Haryana, India
Munish Garg
Faculty of Pharm. Sciences, M.D. University, Rohtak, Haryana, India
Monika Gupta
A.S.B.A.S.J.S.M. College of Pharmacy, Bela (Ropar), Punjab, India