Review Article
Actinomycetes and Drug-An Overview
Department of Microbiology, Jamal Mohammed College, Tiruchirapalli, Tamilnadu, India
P. Prabakarana
Department of Microbiology, PRIST University, Thanjavur, Tamilnadu, India
The antibiotic research from the discovery of Fleming to our days has been a fascinating, exciting, continuously changing and developing adventure. As a result of the frenzied research of the past more than 50 years, in our days ten thousands of natural products derived from microbial sources are known. Interest towards the field were generally increasing, although sometimes declining, interest and the whole story shows some cyclic features with successes and failures and evolved around changing clinical needs and new enabling technology. After the revolution in the “heroic” or “golden era”, in the forties and early fifties, when almost all groups of important antibacterial antibiotics (tetracyclines, cephalosporins, aminoglycosides, macrolides) were discovered, the success story had continued. It seemed in the fifties and sixties that the main problems of chemotherapy had been solved. Antibiotics discovered in this period were mainly isolated from Streptomyces species, representing some 70 to 80% of the all isolated compounds. They were primarily active against bacteria and fungi. In this period the discovery of antitumor, antiviral and non antibiotic-enzyme inhibitory-metabolites, had just started. Most microbiologists distinguish two groups of antimicrobial agents used in the treatment of infectious disease: antibiotics, which are natural substances produced by certain groups of microorganisms, and chemotherapeutic agents, which are chemically synthesized. A hybrid substance is a semi synthetic antibiotic, where in a molecular version produced by the microbe is subsequently modified by the chemist to achieve desired properties. Furthermore, some antimicrobial compounds, originally discovered as products of microorganisms, can be synthesized entirely by chemical means. In the medical and parmaceutical worlds, all these antimicrobial agents used in the treatment of disease are referred to as antibiotics, interpreting the word literally. Although other antibiotics, and their antibiotic affect the organisms may be effective against closely related strains. In most cases, how or why bacteria are resistant to their own antibiotics is also unknown, but it may be worth pondering or studying if we are to understand the cellular and molecular basis of drug resistance in pathogens. Raja et al. (2010a) reported that new antibiotics active against resistant bacteria are required. Bacteria live on earth for several billion years. During this time, they encountered by range of naturally occurring antibiotics. To survive, bacteria developed antibiotics resistance mechanism (Hoskeri et al., 2010). The modern era of antimicrobial chemotherapy began in 1929, with Fleming's discovery of the powerful bactericidal substance, penicillin and Domagk's discovery in 1935 of synthetic chemicals (sulfonamides) with broad antimicrobial activity (Todar, 2009). Actinomycetes are mostly distributed wide range of environment like terrestrial and marine. The presence of extreme environment specially at cryophilic region also reported by Raja et al. (2010b).
TRENDS IN DRUG DISCOVERY
The process by which a single new medicine is developed from a research-based concept into a fully marketed product is lengthy and extremely costly. As a matter of fact, it takes as much as 15 years to complete and is currently estimated to cost around a billion dollars to develop a new drug or therapy. The overestimated factors that contribute to these costs are the fact that the vast majority of the drugs fail at some stage of the development process. Indeed, 10000 compounds need to be investigated at the discovery stage in order to deliver a single new medicine for the market. Importantly, over a quarter of the invested money is spent on the discovery stage of drug development. In addition to the investment and internal R&D, companies are more and more aware that health-based R&D has expanded beyond the proficiencies of any single company. The need for externalization is stronger than ever because it gives the opportunities to access new technologies and novel therapies efficiently. As a result, many companies have started industry collaborations, hence benefiting from complementary expertise and skills (Biotech and Pharmaceutical alliances). Forty five percent of the total drugs approval by FDA in 2006 and 2007 were from the biotech industry. However, the rate of failure was high, 60% failure rate in phase III. In the pharmaceutical industry, the rate of failure was lower, 16% failure rate due to phase III. However, the number of drugs approval by FDA coming from the pharma industry only represented only 35% of total FDA drug approvals (Table 1).
| Table 1: | Drug development scorecard 2006-2007 |
 | |
| Values mentioned in parenthesis are percentage | |
Twenty percent of all drugs approval by FDA comes from collaborations or acquisitions, with a rate of 50% failure rate in phase III. Therefore, the data from the last few years show that the pharmaceutical itself have showed low productivity in proportion to the total number of drugs approval and while the biotech industry show higher number of drug approvals by FDA, the rate of failure is extremely high, leading to less interest by investors. Hence, biotech and pharma collaborations could lead to a better productivity and a smaller failure rate in phase III (Czerepak and Ryser, 2005).
ROLE OF STREPTOMYCETES
Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae (Kampfer, 2006). Over 500 species of Streptomyces bacteria have been described by Euzeby (2008). As with the other Actinobacteria, streptomycetes are gram-positive and have genomes with high GC-content (Madigan and Martinko, 2005). Found predominantly in soil and decaying vegetation, most streptomycetes produce spores and are noted for heir distinct "earthy" odor which results from production of a volatile metabolite, geosmin. Streptomycetes are characterised by a complex secondary metabolism. They produce over two-thirds of the clinically useful antibiotics of natural origin (e.g., neomycin, chloramphenicol) (Kieser et al., 2000). Streptomyces-derived antifungals tend to be macrolide polyenes (large ring structure with lots of conjugated carbon-carbon double bonds) and include such illustrious members as: nystatin (the first Actinobacteria-sourced human antifungal, made by S. noursei), amphotericin B (made by S. nodosus, originally isolated from a sample of Venezuelan soil) and natamycin (made by S. natalensis). There are a friggin’ tonne of Streptomyces-derived antibiotics used specifically as antibacterial agents. To begin with, a good number of the aminoglycosides (a class of antibiotics that possess cyclohexyl rings substituted with amine groups and linked together by glycosidic bonds) are the work of this genus. These include Streptomycin by S. griseus, neomycin and kanamycin, respectively produced by S. fradiae and S. kanamyceticus. Other antibacterial antibiotics of note include: erythromycin (a macrolide that often subs for penicillin when people be allergic to it, made by S. erythraea), tetracycline (a longstanding acne drug that makes you light-sensitive, made by S. rimosus), chloramphenicol (cheap, effective, but can cause aplastic anemia, made by S. venezuelae), vancomycin (a relatively ginormous glycopeptide that can turn people red, made by S. orientalis) and thienamycin (made by S. cattleya, modified by us to make imipenem, the first carbapenem beta-lactam antibiotic). A number of the antibiotics produced by Streptomyces have proven to be too toxic for use as antibiotics in humans, but because of their toxicity towards cells (specifically dividing cells) they have been reinvented as chemotherapy drugs. We’re talking drugs like: actinomycin-D (the original), bleomycin (glycopeptide made by S. verticullus), mitomycin (aziridine made by S. lavendulae) and plicamycin (made by S. plicatus) (Birnbaum et al., 1985). Plus we have the anthracyclines daunorubicin and doxorubicin (S. peucetius and migrastatin (macrolide, under investigation, made by S. platensis).
ACTINOMYCETES AND APPROVED DRUGS
Tigecycline is the 9-tert-butyl-glycylamido derivative of minocycline, which is a semi-synthetic product of chlortetracycline isolated from Streptomyces aureofaciens. Tigecycline exhibited antibacterial activity typical of other tetracyclines, but with more potent activity against tetracycline-resistant organisms. Tigecycline is only utilized in an injec formulation for clinical use, unlike currently marketed tetracyclines that are available in oral dosage forms (Zhanel et al., 2004).
Everolimus is an orally active 40-O-(2-hydroxyethyl) derivative of rapamycin, originally produced from Streptomyces hygroscopicus. Everolimus exhibits its immunosuppressive effect by blocking growth factor (interleukin (IL)-2 and IL-15) mediated proliferation of hematopoietic (T cells and B cells) and non-hematopoietic (vascular smooth muscle cells) cells through inhibiting p70 S6 kinase. This leads to arrest of the cell cycle at the G1/S phase (Chapman and Perry, 2004)
Telithromycin is a semi-synthetic derivative of the 14-membered macrolide, erythromycin A, isolated from Saccharopolyspora erythraea and retains the macrolactone ring as well as a D-desosamine sugar moiety. It inhibits protein synthesis by interacting with the peptidyltransferase site of the bacterial 50S ribosomal subunit and exhibits antibacterial effect on respiratory tract pathogens resistant to other macrolides (Zhanel et al., 2002).
Miglustat has been approved for patients unable to receive enzyme replacement therapy as a therapeutic drug for type I Gaucher disease. Miglustat, an analog of nojirimycin isolated from the broth filtrate of Streptomyces lavendulae, reversibly inhibits glucosylceramide synthase, a ceramide-specific glucosyltransferase that catalyzes the formation of glucocerebrosid and thereby decreases tissue storage of glucosylceramide. Gaucher disease is a progressive lysosomal storage disorder associated with pathological accumulation of glucosylceramide in cells of the monocyte/macrophage lineage. Enzyme replacement therapy using human placenta-derived alglucerase (Ceredase) has been available for type I Gaucher disease (Pastores et al., 2005).
Daptomycin is a cyclic lipopeptide antibacterial agent derived from Streptomyces roseosporus, which has been approved for the treatment of complicated skin and skin structure infections (cSSSIs). Daptomycin binds to bacterial cell membranes and then disrupts the membrane potential, leading to blocking of the synthesis of proteins, DNA, and RNA (Fenton et al., 2004).
Amrubicin hydrochloride is a completely synthetic 9-aminoanthracycline and converts to its active form in the body. Amrubicin, a derivative of doxorubicin isolated from Streptomyces peucetius var caesius, demonstrated activity comparable to that of doxorubicin on transplan animal tumors, including P 388 leukemia, sarcoma 180 and Lewis lung carcinoma, and more potent antitumor activity against human tumor xenografts of breast, lung and gastric cancer (Sugiura et al., 2005).
Biapenem is a new analog of carbapenem based on thienamycin, isolated from Streptomyces cattleya, an antibacterial agent effective against both Gram-negative and Gram-positive bacteria including species producing β-lactamases. Biapenem is more s to hydrolysis by human renal dehydropeptidase-I than imipenem, meropenem, and panipenem. The early carbapenems (eg., imipenem) are not s to hydrolysis by human renal dihydropeptidase-I (DHP-I) and consequently are coadministered with a DHP-I inhibitor (eg., cilastatin). Biapenem can be administered as a single agent without a DHP-I inhibitor (Perry and Ibbotson, 2002).
Ertapenem is a new 1β-methylcarbapenem based on thienamycin, isolated from Streptomyces cattleya, with broad-spectrum antibacterial activity and improved stability to hydrolysis by renal dehydropeptidase enzymes located in the brush border of the kidneys (Sader and Gales, 2001). Ertapenem exhibits excellent antibacterial activity against clinically relevant Enterobacteriaceae including Escherichia coli, Klebsiella sp., Citrobacter sp., Enterobacter sp., Morganella morganii, Proteus sp. and Serratia marcescens.
Pimecrolimus is a novel analog of ascomycin, isolated as a fermentation product of Streptomyces hygroscopicus var ascomyceticus. Its mechanism of action involves blocking T cell activation via the pimecrolimus-macrophilin complex that prevents the dephosphorylation of the cytoplasmic component of the nuclear factor of activated T cells (NF-AT). This drug was approved for the treatment of inflammatory skin diseases such as allergic contact dermatitis and atopic dermatitis (Gupta and Chow, 2003).
Gemtuzumab ozogamicin is a prodrug of calicheamicin bound to anti-CD33 monoclonal antibody. The calicheamicins (also known as the LL-E3328 antibiotics) were discovered from fermentation products produced by Micromonospora echinospora sp. calichensis. Lysosomes in the cells cleave the covalent link between the monoclonal antibody and calicheamicin, allowing calicheamicin release. Calicheamicin is a hydrophobic member of the enediyne family of DNA-cleaving antibiotics and effective in treatment of patients with acute myeloid lymphoma (Lee et al., 1987).
DRUGS IN CLINICAL TRIALS
Elsamitrucin (elsamicin A), which has a common chromophore with chartreusin from Streptomyces chartreusis, was isolated from the unidentified actinomycete strain J 907-21. This compound binds to DNA but also inhibits activity of topoisomerase II, leading to an antitumor effect (Portugal, 2003). Brostallicin an α-bromoacryloyl derivative of distamycin A that was isolated from the culture mycelium of Streptomyces distallicus, is a DNA minor groove binding anticancer agent (Broggini et al., 2004). Its mechanism of action is associated with activation after binding to glutathione (GSH), catalyzed by glutathione-S-transferase (GST) and the relatively high GST/GSH levels of cancer cells have made them more susceptible to the antitumor effects of brostallicin than normal cells (Geroni et al., 2002) Geldanamycin, a polyketide natural product, was originally obtained from Streptomyces hygroscopicus (Sasaki et al., 1970) and its analogs [17-AAG and 17-DMAG] are currently under clinical evaluation due to their inhibition of the protein chaperone Heat Shock Protein (HSP) 90 (Bisht et al., 2003).
DISTRIBUTION OF BIOACTIVE NATURAL PRODUCTS
Almost all types of living things can produce antibiotics and similar natural products, being secondary metabolites. They are produced by prokaryotic (Prokaryote, Monera) and eukaryotic organisms belonging to the Plant and Animal Kingdom, alike. The secondary metabolite producing ability, however, is very uneven in the species of living world. In the Prokaryote and Plant Kingdom there are distinct groups of organisms, namely unicellular bacteria, eukaryotic fungi and first of all filamentous actinomyces being the most frequent and most versatile producers. In the group of prokaryotic, unicellular bacteria the Bacillus and Pseudomonas species are the most frequent producers. In the recent years Myxo- and Cyanobacteria species seem to join to these distinguished organisms as prolific species. Mycobacteria, Mycoplasmatales and Spirotheces are far less frequent producers. The total number of known bioactive compounds in this group is about 3800; 17% of all microbial metabolites. The filamentous Actinomycetales species produces over 10000 bioactive compounds, 7600 derived from Streptomyces and 2500 from the so called rare actinomycetes (rare actino) species, represent the largest group (45%) of bioactive microbial metabolites. Among the eukaryotic microscopic fungi the producing capability of imperfect fungi, the ascomycetes and several other filamentous and endophytic funga l species are the most significant. The basidiomycetes are also frequently reported producers, while yeasts, phycomycetes, slime mould srarely produce bioactive metabolites. The total number of bioactive fungal product is approximately 8600, representing 38% of all microbial products ( Table 2).
| Table 2: | Approximate number of bioactive microbial natural products (2002) |
 | |
From the known (altogether 22500) antibiotics and similar bioactive microbial compounds, less than one percent, only about 150 compounds, is in direct use in the human and veterinary medicine, and agriculture. In the human therapy about one hundred compounds, most of them derived from actinomycetales species, are in direct practical use.
THE RESEARCH OF MICROBIAL METABOLITES
Obviously various actinomycetales, first of all the Streptomyces species and filamentous fungi, and to a lesser extent several bacterial species are the most noteworthy producers both in respect of numbers, versatility and diversity of structures of the produced metabolites. The significance and frequency of these main types of microbes as producers of bioactive metabolites had varied significantly during the last decades. In the beginning of the antibiotic era the fungal (Penicillin, Griseofulvin) and bacterial (Gramicidin) species were in the foreground of the interest, but after the discovery of streptomycin and later chloramphenicol, tetracyclines and macrolides the attention turned to the Streptomyces species. In the fifties and sixties the majority (70%) of antibiotics were discovered from these species. In the next two decades the significance of the non-Streptomyces actinomycetales species (rare actinos) were increased, up to a 25-30% share of all antibiotics. From the early nineties the number of bioactive compounds isolated from various filamentous and other microscopic and higher fungal species had continuously increased up to more than 50% by the turn of the millennium (2000). The interest to bacteria in the recent years had only slightly increased. Simultaneously, the ratio of actinomycetal compounds naturally had definitely decreased. The most characteristic and a little bit surprising feature of the recent years just is this declining representation of the formerly exhaustively investigated actinomycetes. Their share among all microbial products presently is only 30-35%, in contrast to the 75-80% share from the sixties to eighties. The shift in their apparent participation, is rather the result of the favoured fashion of the fungal screening in some laboratories. Presently the claim for new microbial pharmacophores led to the shift of the efforts in most places towards the discovery of fungal products from the large pool of untapped fungal world. However, the present slight over-estimation of the capability of fungi is rather a periodic phenomenon (Donadio et al., 2002a). It is very likely that the changes in the interest towards the favorite microbes (as happened with the actinomycetes in the earlier years) depends on new expectations, the changing needs in the human therapy, and probably sometimes on fashion. It is also likely that for numerous reasons the actinomycetales, besides the fungi, will remain equally important and promising producers in the future.
Notwithstanding the recent drop, the predominant part, 45% of the presently known bioactive microbial metabolites, over 10000 compounds were still isolated from various actinomycetales species, 34% from Streptomyces and 11% from the rare actinomycetes. The most frequent producers, the Streptomyces species produces 7600 compounds (74% of all actinomycetales), while the rare actinomycetes represent 26%, altogether 2500 compounds. The representation of rare actino products in 1970 was only 5%.
| Table 3: | Number of actinomycetales species producing bioactive microbial metabolites |
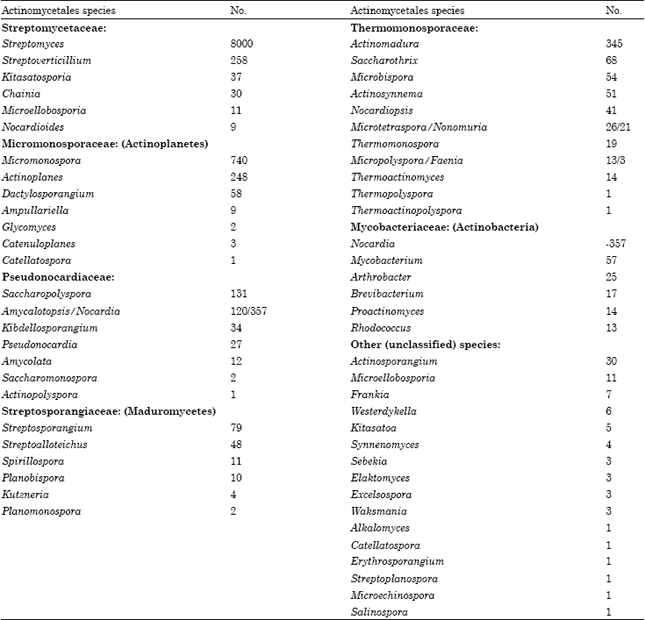 | |
In this group Micromonospora, Actinomadura, Streptoverticillium, Actinoplanes, Nocardia, Saccharopolyspora and Streptosporangium species are the most frequent producers, each produces several hundreds of antibiotics. In Table 3 the numbers of actinomycetales species, including the all rare actinos, known to produce bioactive metabolites, are summarized. These fastidious organisms, the rare actinos, produce perhaps the most diverse and most unique, unprecedented, sometimes very complicated compounds exhibiting excellent antibacterial potency and usually low toxicity. It is interesting that several chemical types, such as simpleterpenoids or benzenoids are almost completely absentfrom this compounds. In this group of metabolites there are numerous practically very important compounds such as gentamicins, erythromycins, vancomycin, or rifamycin. Numerous recently introduced chemotherapeutic and agricultural agents (ziracin, dalbavacin, spynosin), are also rare actinomycetes products.
| Table 4: | Bioactivity types of microbial metabolites numbers of discovered bioactivities |
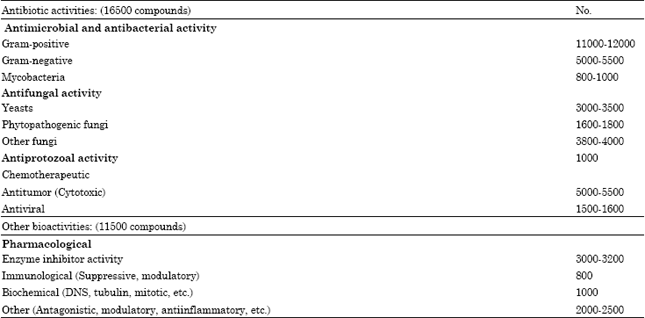 | |
It is noteworthy that the vancomycinristocetin type complicated glycopeptides are produced almost exclusively by various rare actino species. Presently more than 50 rare actinomycetes are known as producers of the 2500 bioactive compounds, but in 1970 only 11 rare Actinomycetes species, producing altogether 50 compounds, were known. The number of all taxonomically described rare actinomycetes today is close to 100 but this number, due to the recently developed genetic and isolation techniques will, be in all means quickly increasing (Donadio et al., 2002b). Most of the actinomycetes including non streptomyces were found to be antibiotic produces (Table 3).
BIOACTIVITIES OF SECONDARY METABOLITES
The presently known secondary microbial metabolites, exhibit a great numbers of diverse and versatile biological effects, first of all antimicrobial activities. In the scientific literature already hundreds of different pathogenic and other microbes (Gram-positive, Gram-negative bacteria, fungi, yeasts, etc. are described as test organisms in the direct activity-based screenings. The most frequent test organisms were Bacillus subtilis, Staphylococcus aureus, Micrococcus (Sarcina) lutea, Escherichia coli, Pseudomonas aeruginosa, Saccharomyces cerevisiae, Candida albicans and others. Antiviral tests, inhibition of viral enzymes, activities connecting with neoplastic diseases from simple cytotoxicity assay methods (P-388, KB, L-1210 cell lines) to angiogenesis inhibition, etc., are used most frequently for detection of other, non antimicrobial activities of metabolites (Table 4). However, the infectious diseases are mainly treated with natural antibiotics and their derivatives, while still the majority of drugs applied in the so-called physiological diseases are synthetic products. The challenge for natural medical products to treat these diseases is huge. There is an urgent need in this area to identify totally new active chemotypes at least as lead compounds for effective drug development.
The total number of the microbial metabolites recognized until now is around 50000 and the number of all known natural products is around one million most of them derived from Actinomycetes. It is an obvious question, where is the border in the diversity of natural products? Where is the limit or is there any limit at all, in the continuous increase of the number of new microbial or natural compounds The reinvestigation of the known natural/microbial products and especially the whole microbial population (natural products and microbiological libraries) with at wide variety, more selective, sensitive specific methods, especially in the light of the expanding knowledge of microbial genetics and the acquired knowledge about various genomes, would be especially fruitful. In all means,in the future we will discover more and more new functions, new activities of the microbial metabolites, will understand their real role and function and will expand the area of their practical utilization.