Research Article
Molecular Modelling Analysis of the Metabolism of Udenafil
Discipline of Biomedical Science, School of Medical Sciences, Faculty of Medicine, Cumberland Campus, C42, The University of Sydney, Lidcombe, NSW, Australia
Udenafil (UDF; DA-8159; 5-[2-propyloxy-5-(1-methyl-2-pyrrollidinyl-ethylmidosulfonyl) phenyl]-1-methyl-1,6-dihydro-7H-pyrazolo(4,3-d)pyrimidin-7-one) is a potent and selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDER5), developed for the treatment of male erectile dysfunction (Hegde and Schmidt, 2006; Choi et al., 2002). Inhibition of PDER5 results in an increase in the concentration of endogenous cGMP in the penile corpus cavernosum. cGMP induces smooth muscle relaxation and the subsequent increase in blood flow leading to a sustainable erection. Unlike sildenafil, UDF does not inhibit PDE11, which has been implicated in myalgia and testicular toxicity (Lee et al., 2005). UDF has a rapid onset with a long duration of action (Hegde and Schmidt, 2006). The drug is found to produce up to 91% vaginal penetration success rate and to 67% intercourse completion rate compared to 29% completion rate in placebo.
In rat microsomes, UDF is metabolized to three major metabolites namely N-dealkylatedudenafil (NDAUDF), hydroxyudenafil (UDFM1) and N-demethyludenafil (UDFM3) (Choi et al., 2002). The predominant active metabolite in humans is NDAUDF, generated via CYP3A4 metabolism so that there is the possibility of drug-drug interactions when the drug is administered concomitantly with known inhibitors of CYP3A4. Following oral administration, plasma concentrations of NDAUDF are found to be about 50% of those seen for the parent drug UDF (Kim et al., 2003). Figure 1 gives the proposed metabolic pathway for UDF in rat liver preparations. The pharmacological activity of NDAUDF in terms PDER5 inhibitory activity is about a half that of UDF (Kim et al., 2005). UDF is found to be a very weak inhibitor of CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4, with most inhibition occurring against CYP2D6 (Ji et al., 2004).
The most frequent adverse effects of UDF are mild-to-moderate facial flushing and headache (Hegde and Schmidt, 2006). In present study, molecular modelling analyses have been carried out using the program (Spartan, 2004) to investigate the relative stability of RMT and its metabolites with the aim of providing a better understanding of their relative toxicity.
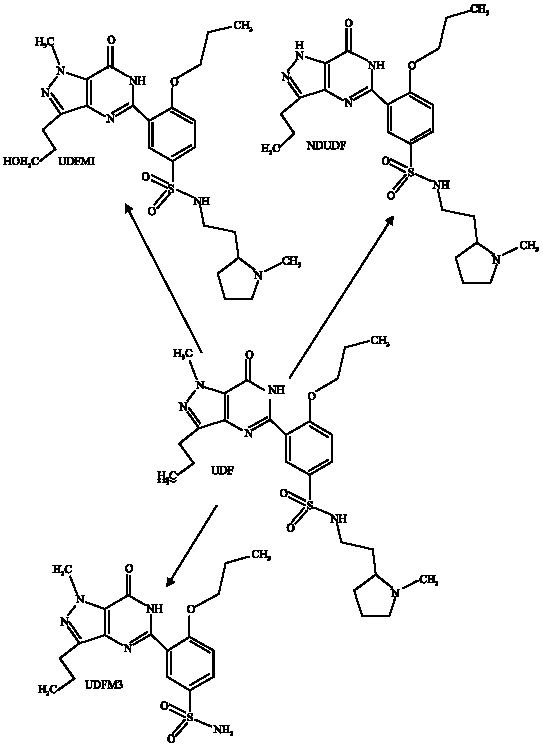 | |
| Fig. 1: | Metabolic pathways for DA-8159 (Choi et al., 2002) |
The study was carried out in the Discipline of Biomedical Science, Faculty of Medicine, The University of Sydney during January to April 2007. Previous studies have shown that xenobiotics and their metabolites which are kinetically labile and abound in electron-deficient regions on the molecular surface tend to induce cellular toxicity due to glutathione depletion and cause DNA damage due to oxidation of nucleobases in DNA (Huq, 2006a, b).
COMPUTATIONAL METHODS
The geometries of UDF and its metabolites UDFM1, NDAUDF and UDFM3 were optimised based on molecular mechanics, semi-empirical and DFT calculations, using the molecular modelling program Spartan `02. Molecular mechanics calculations were carried out using MMFF force field. Semi-empirical calculations were carried out using the routine PM3. Density Functional theory (DFT) calculations were carried at B3LYP/6-31G* level. In optimization calculations, a RMS gradient of 0.001 was set as the terminating condition. For the optimised structures, single point calculations were carried out to give heat of formation, enthalpy, entropy, free energy, dipole moment, solvation energy, energies for Highest Unoccupied Molecular Orbital (HOMO) and Lowest Unoccupied Molccular Orbital (LUMO). The order of calculations: molecular mechanics followed by semi-empirical followed by DFT ensured that the structure was not embedded in a local minimum. To further check whether the global minimum was reached, some calculations were carried out with improvable structures. It was found that when the stated order was followed, structure corresponding to the global minimum or close to that could ultimately be reached in all cases. Although RMS gradient of 0.001 may not be sufficiently low for vibrational analysis, it is believed to be sufficient for calculations associated with electronic energy levels (Huq and Alsheri, 2006).
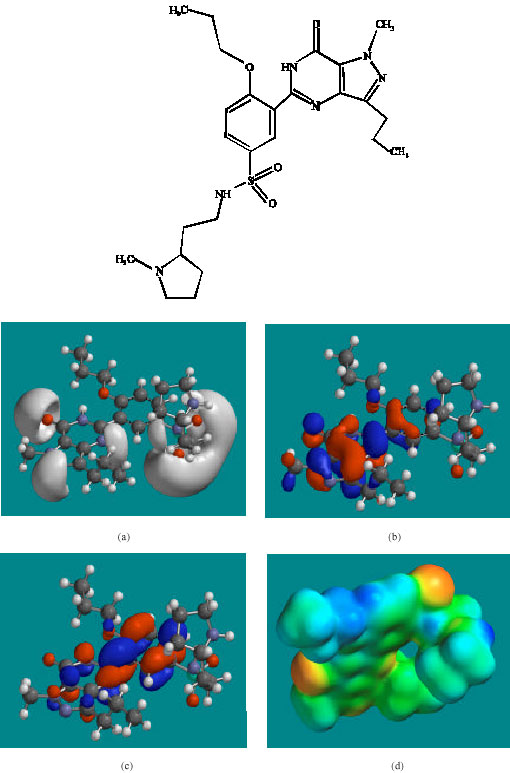
Table 1 gives the total energy, heat of formation as per PM3 calculation, enthalpy, entropy, free energy, surface area, volume, dipole moment and energies of HOMO and LUMO as per both PM3 and DFT calculations for UDF and its metabolites UDFM1, NDAUDF and UDFM3. Figure 2-5 give the regions of negative electrostatic potential (greyish-white envelopes) in (a), HOMOs (where red indicates HOMOs with high electron density) in (b), LUMOs in (c) and density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) in (d) as applied to the optimised structures of UDF and its metabolites UDFM1, NDAUDF and UDFM3.
All the metabolites of UDF are found to have higher solvation energies than the parent drug, indicating that the metabolites would have a greater solubility in water and hence more easily excreted in the urine.
| Table 1: | Calculated thermodynamic and other parameters of UDF and its metabolites |
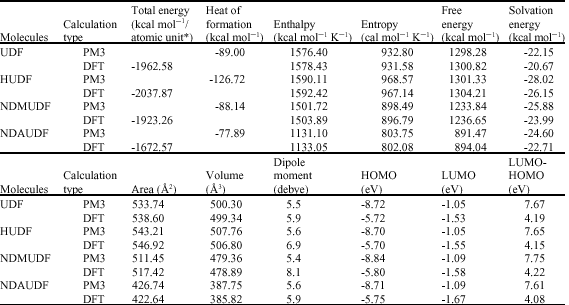 | |
| *: In atomic units from DFT calculations Fig. 2: Structure of UDF giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) | |
 | |
| Fig. 2: | Structure of UDF giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
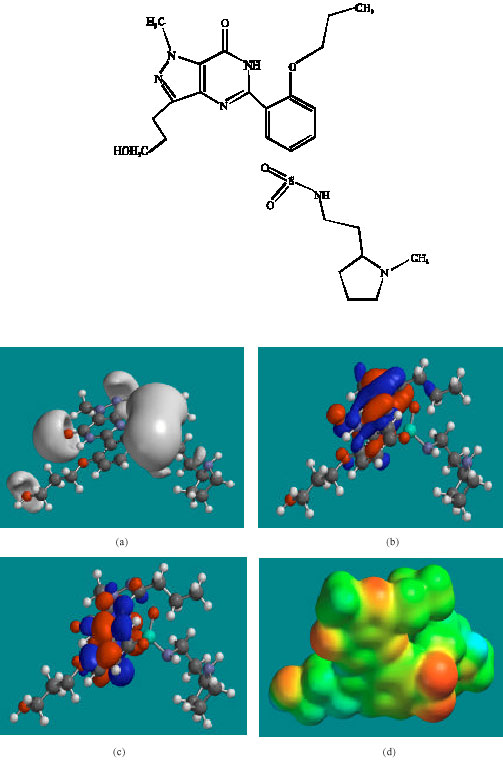 | |
| Fig. 3: | Structure ofUDFM1 giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
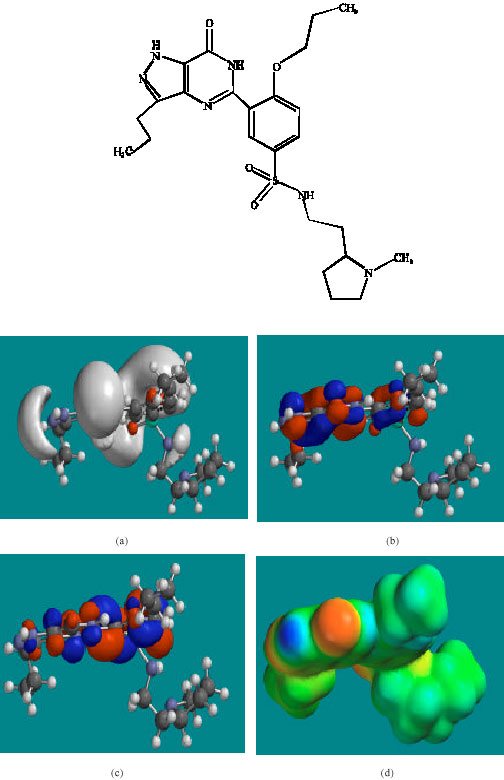 | |
| Fig. 4: | Structure of NDAUDF giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
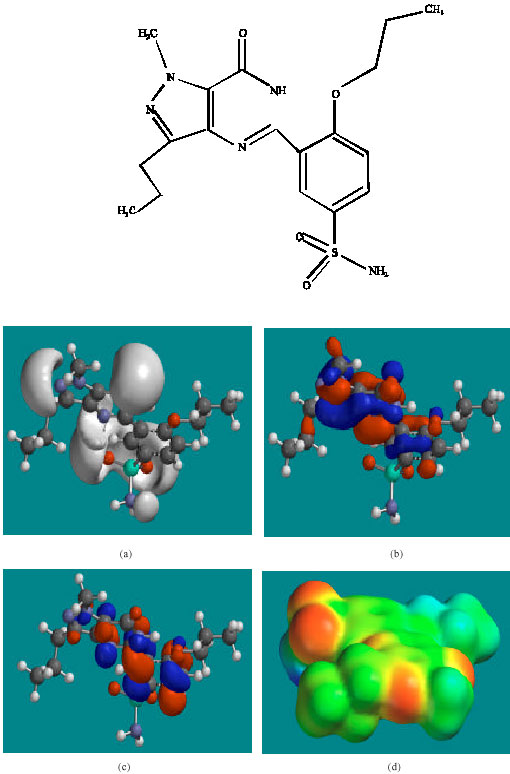 | |
| Fig. 5: | Structure of UDFM3 giving in: (a) the electrostatic potential (greyish envelope denotes negative electrostatic potential), (b) the HOMOs, (where red indicates HOMOs with high electron density) (c) the LUMOs (where blue indicates LUMOs) and in (d) density of electrostatic potential on the molecular surface (where red indicates negative, blue indicates positive and green indicates neutral) |
In the case of UDF, UDFM1, NDAUDF and UDFM3, both the HOMOs with high electron density and the LUMOs are found to be close to mainly the non-hydrogen atoms of the pyrazole, pyrimidine and phenyl rings. The overlap of HOMO with high electron density and region of negative electrostatic potential at some positions, gives further support to the idea that the positions may be subject to electrophilic attack.
The molecular surfaces of UDF and NDAUDF are found to abound in neutral green and electron-deficient blue regions so that they can be subject to lyophilic and nucleophilic attacks. The latter attack can be due to glutathione and nucleobases in DNA so that the two compounds may induce cellular toxicity due to glutathione depletion and DNA damage due to oxidation of nucleobases in DNA. The molecular surfaces of UDFM1 and UDFM3 are found to abound in electron-rich red and yellow regions so that the two compounds may be more subject to electrophilic attacks. This means that UDFM1 and UDFM3 may behave more like antioxidants and thus play a protective role.
When the surface area and volume of UDF are compared with those of its metabolites (Table 1), the values for the metabolites including the active metabolite NDAUDF are found to be distinctly different from those of the parent drug so that none of the metabolites.
Udenafil (UDF) is a potent and selective inhibitor of cGMP, used for the treatment of male erectile dysfunction. Modelling analyses based on molecular mechanics, semi-empirical (PM3) and DFT (at B3LYP/6-31G* level) calculations show that UDF and its metabolites have LUMO-HOMO energy differences of the order of 4.1 to 4.2 eV so that the compounds would all be moderately inert kinetically. The molecular surfaces of UDF and NDUDF are found to abound in neutral green and electron-deficient blue regions so that they can be subject to lyophilic and nucleophilic attacks. The latter can be due to glutathione and nucleobases in DNA so that the two compounds may induce cellular toxicity due to glutathione depletion and DNA damage due to oxidation of nucleobases in DNA. The molecular surfaces of UDFM1 and UDFM3 are found to abound in electron-rich red and yellow regions so that the two compounds may be more subject to electrophilic attacks. This means that UDFM1 and UDFM3 may behave more like antioxidants and thus play a protective role.
Fazlul Huq is grateful to the Discipline of Biomedical Science, School of Medical Sciences, The University of Sydney for the time release from teaching.