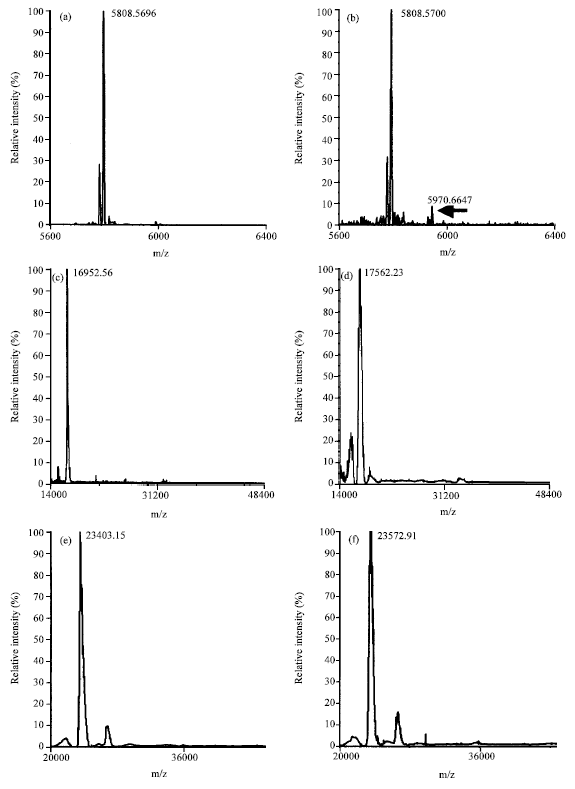
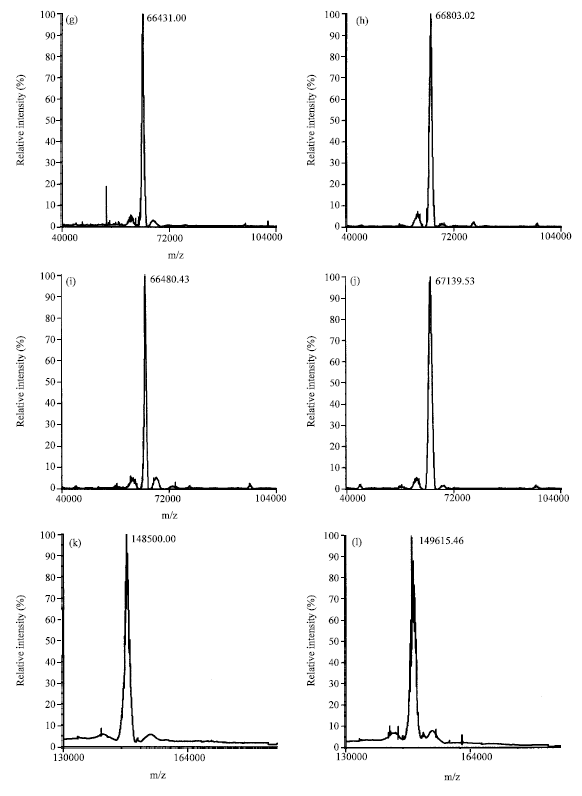
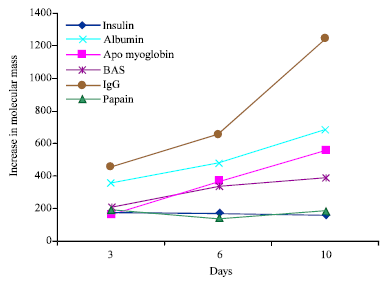
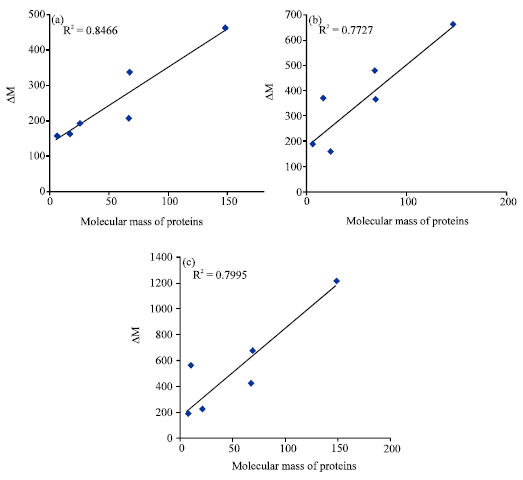
Research Article
Influence of Protein Molecular Mass on the Glycation
Mass Spectrometry and Proteomics Group, Division of Organic Chemistry, National Chemical Laboratory, Pune, India
Mahesh J. Kulkarni
Mass Spectrometry and Proteomics Group, Division of Organic Chemistry, National Chemical Laboratory, Pune, India