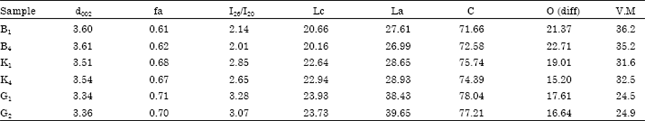
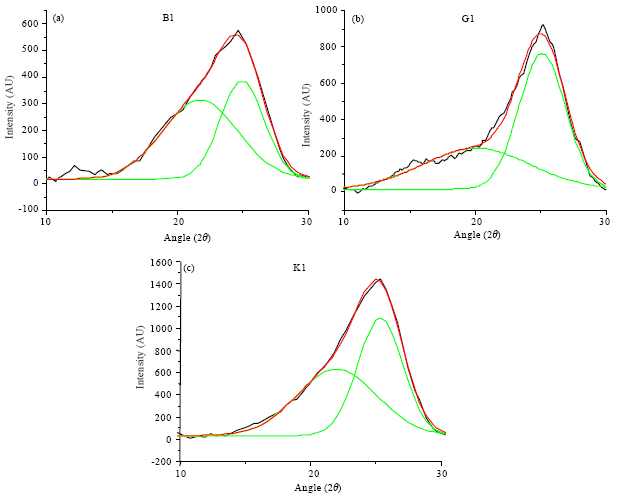
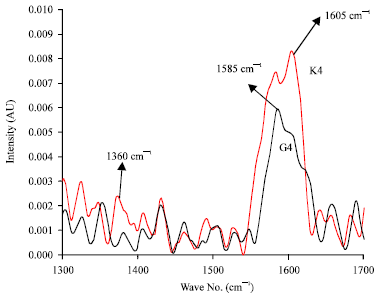
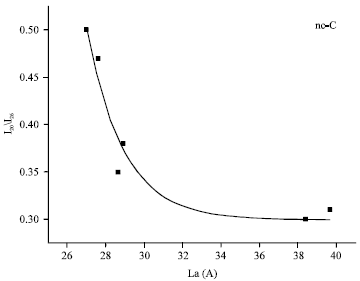
Research Article
Structural Characterization of Selected Indian Coals by X-ray Diffraction and Spectroscopic Techniques
Department of Physics, Christ University, Bangalore-29, Kanataka, India
A.G. Kunjomana
Department of Physics, Christ University, Bangalore-29, Kanataka, India