Research Article
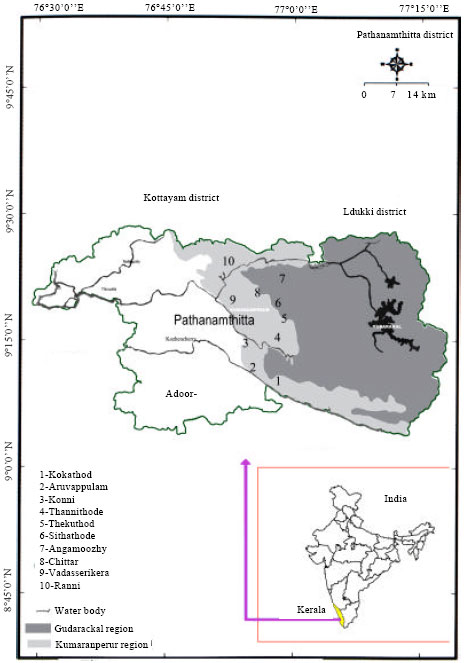
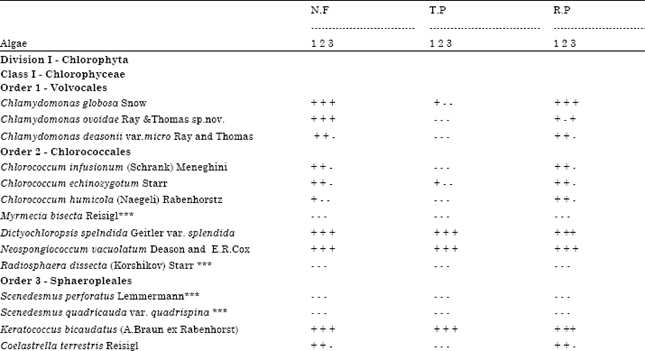
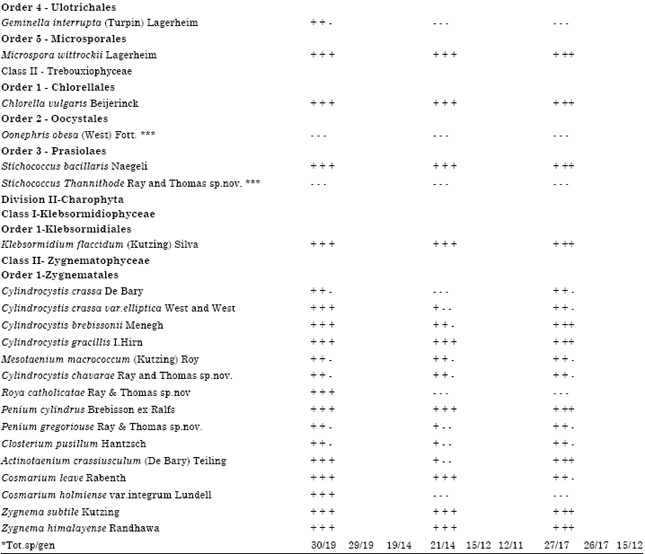
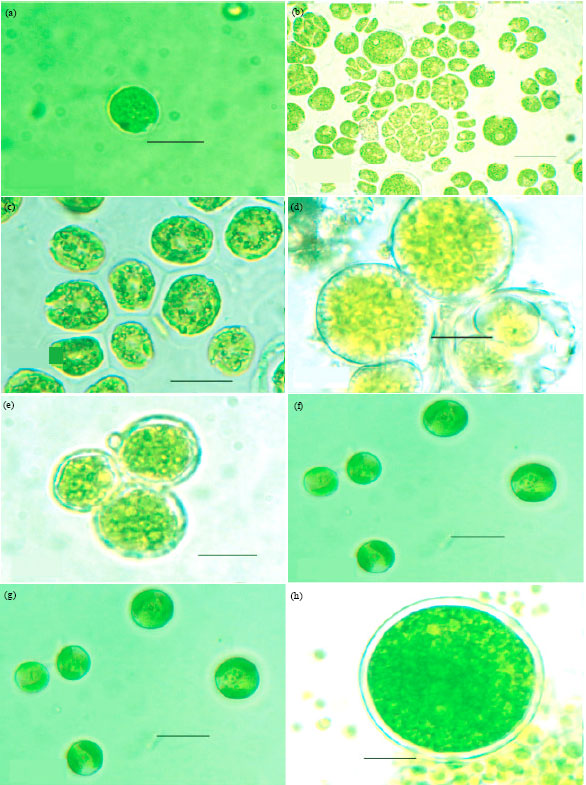
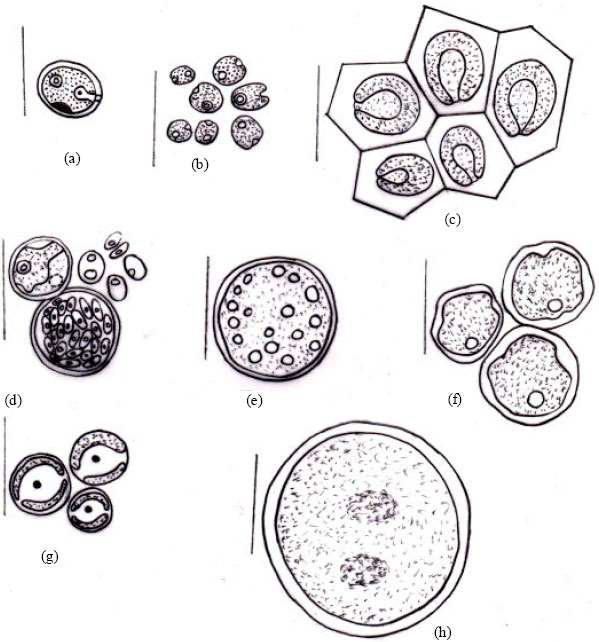
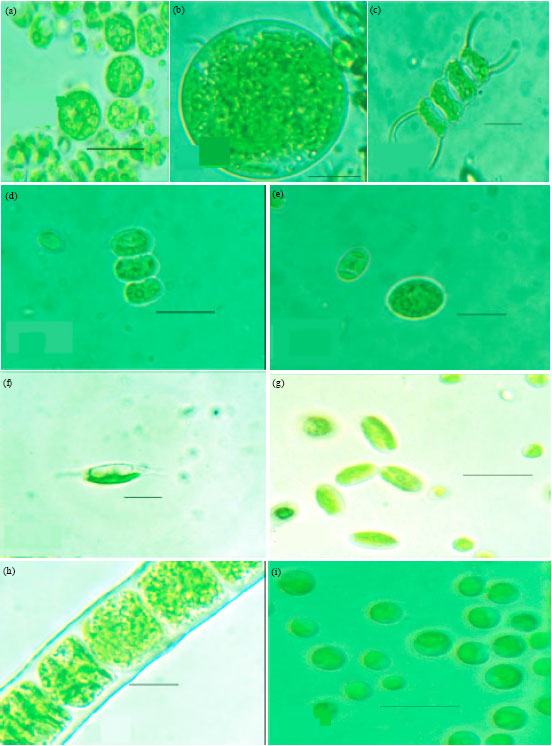
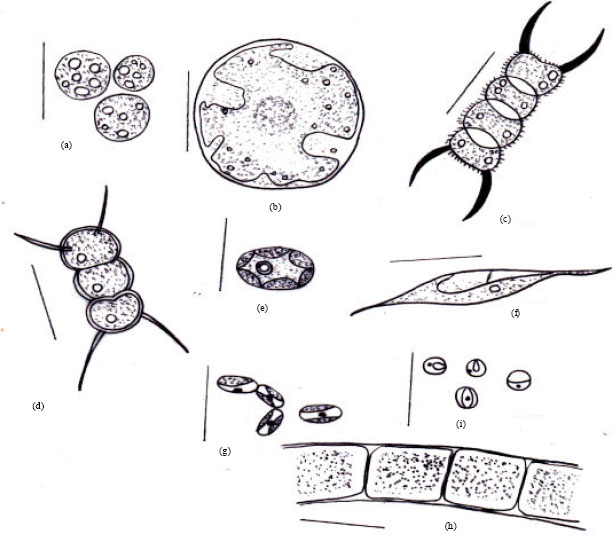
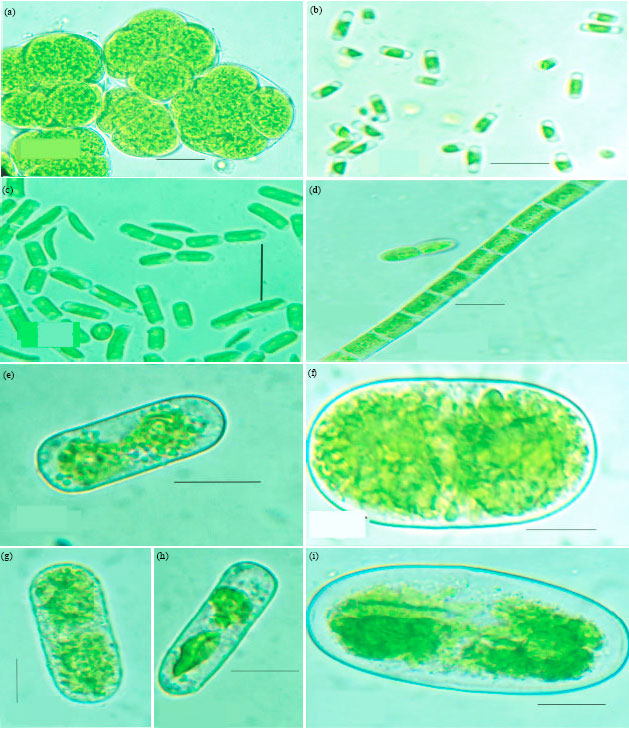
Ecology and Diversity of Green-algae of Tropical Oxic Dystrustepts Soils in Relation to Different Soil Parameters and Vegetation
Research Laboratory of Ecology and Ecotechnology, School of Biosciences, Mahatma Gandhi University, Kottayam, Kerala, 686560, India
T. Binoy Thomas
Environment Science Research Lab, Postgraduate Department of Botany, St Berchmans College, Changanacherry, Kerala, 686 103 India