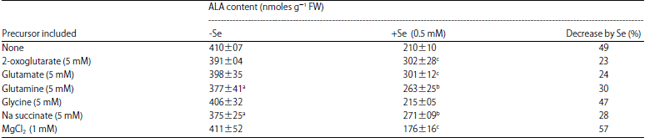
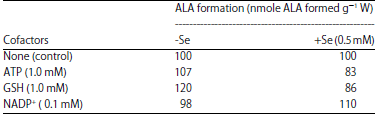
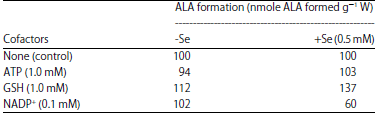
Research Article
Influence of Selenium Supplementation on δ-Aminolevulinic Acid Formation in Greening Maize Leaf Segments
School of Biochemistry, Devi Ahilya Vishwavidyalaya, Takshashila Campus, Khandwa Road, 452 017 Indore (M.P.), India
LiveDNA: 91.14319
Mahija Panwar
School of Biochemistry, Devi Ahilya Vishwavidyalaya, Takshashila Campus, Khandwa Road, 452 017 Indore (M.P.), India
Rekha Gadre
School of Biochemistry, Devi Ahilya Vishwavidyalaya, Takshashila Campus, Khandwa Road, 452 017 Indore (M.P.), India