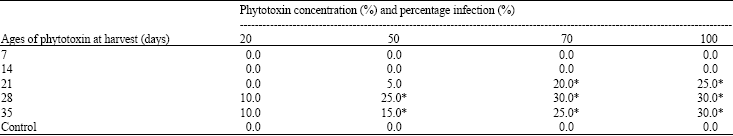
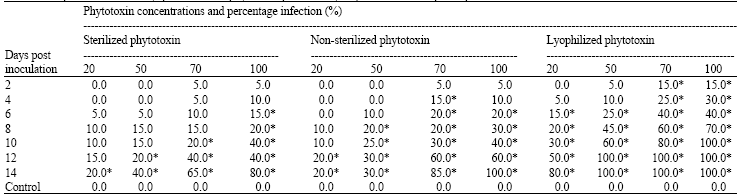
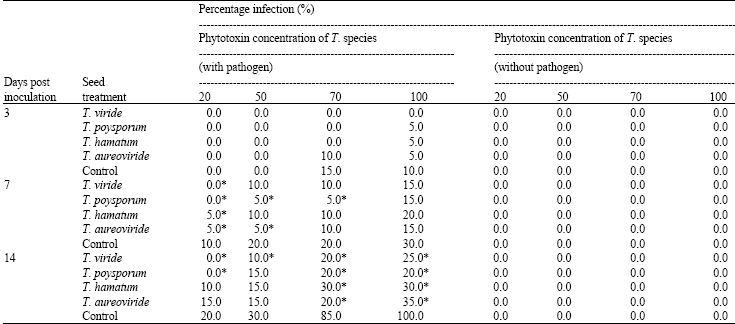
Research Article
Control of Phytotoxin from Ceratocystis paradoxa Using Trichoderma Species Phytotoxins on Oil Palm (Elaeis quineensis Jacq.) Sprouted Seeds
Nigerian Institute for Oil Palm Research (NIFOR), Plant Pathology Division, P.MB. 1030, Benin City, Edo State, Nigeria
I. B. Omamor
Nigerian Institute for Oil Palm Research (NIFOR), Plant Pathology Division, P.MB. 1030, Benin City, Edo State, Nigeria
E. A. Dimaro-Oruade
Nigerian Institute for Oil Palm Research (NIFOR), Plant Pathology Division, P.MB. 1030, Benin City, Edo State, Nigeria
L. A. Ogunkanmi
Department of Cell Biology and Genetics, University of Lagos, Akoka, Yaba, Lagos State, Nigeria