Research Article
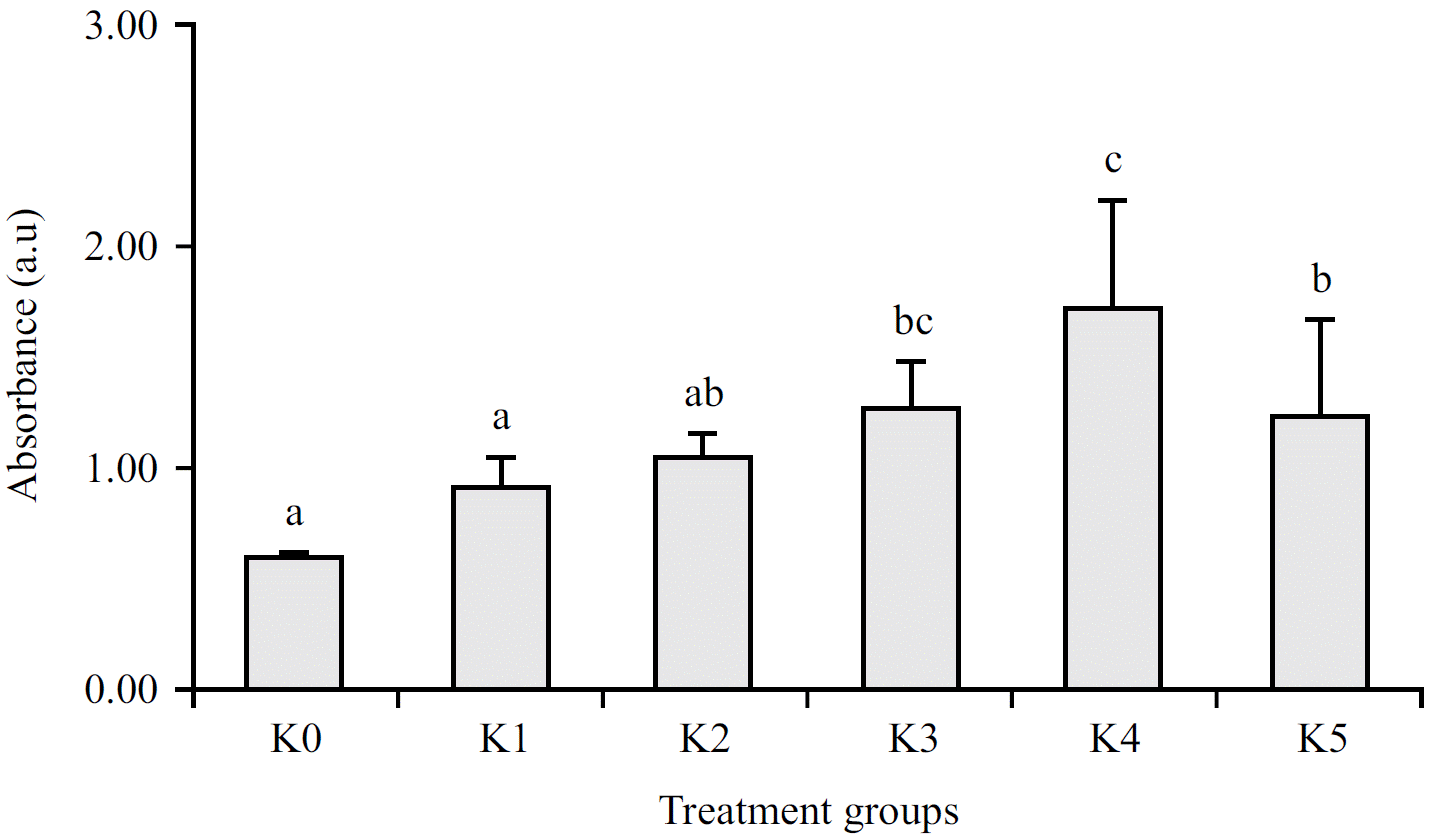
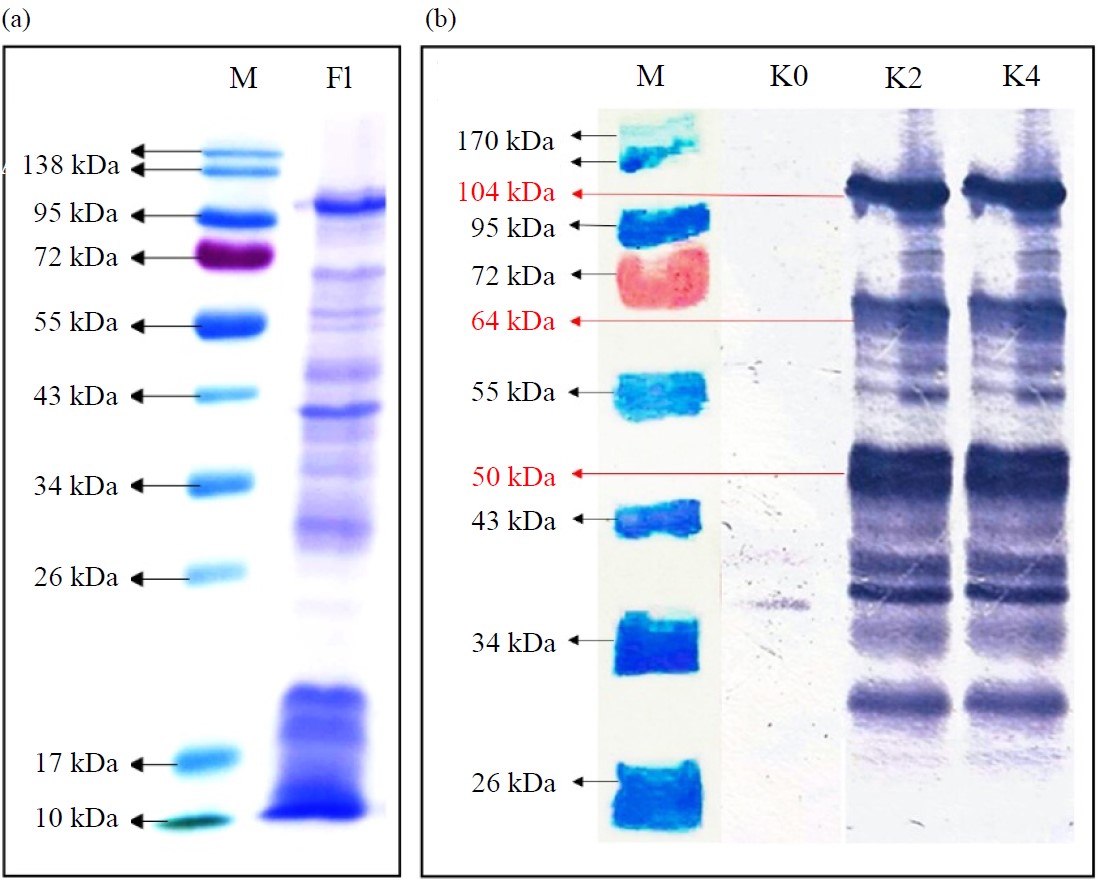
Immunogenicity Response of Mus musculus var. Balb/C after Immunization using Flagellin Salmonella typhi Serovar Semarang
Master Program of Clinical Laboratory Science, Postgraduate School, Universitas Muhammadiyah Semarang, Jl. Kedungmundu Raya No. 18, Semarang, Jawa Tengah 50273, Indonesia
LiveDNA: 62.39768
ORCID: 0000-0002-9415-1955
I. Agus Adi Gunawan
Master Program of Clinical Laboratory Science, Postgraduate School, Universitas Muhammadiyah Semarang, Jl. Kedungmundu Raya No. 18, Semarang, Jawa Tengah 50273, Indonesia
Nastiti Wijayanti
Faculty of Biology, Universitas Gadjah Mada, Jl. Teknika Selatan, Sekip Utara, Bulaksumur, Sleman, Yogyakarta 55281, Indonesia
LiveDNA: 62.32258
ORCID: 0000-0001-6483-2816
Muchamad Dafip
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Negeri Semarang, Jl. Taman Siswa, Kampus Sekaran, Gunung Pati, Semarang, Central Java 50229, Indonesia
LiveDNA: 62.35020
ORCID: 0000-0001-8059-8193