Research Article
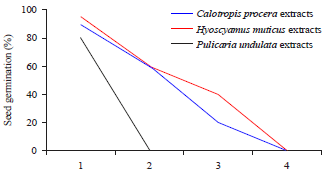
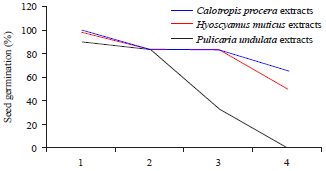
Allelopathic Effect of Calotropis procera, Hyoscyamus muticus and Pulicaria undulata Extracts on Seed Germination of Portulaca oleracea and Chenopodium murale
Department of Biology, University College of Tayma, Tabuk University, Kingdom of Saudi Arabia
LiveDNA: 966.32182