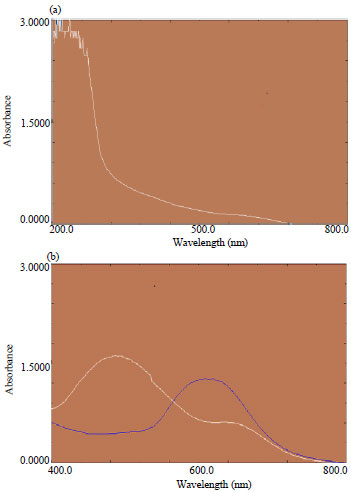
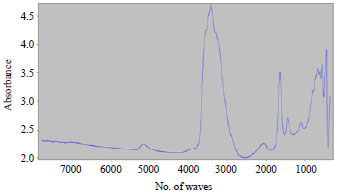
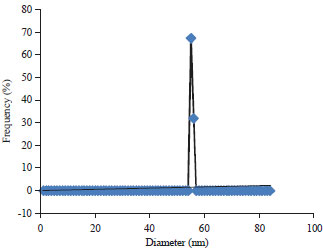
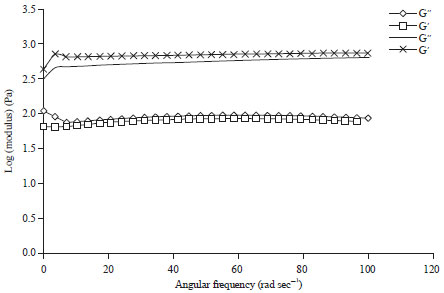
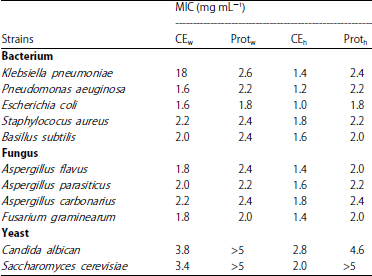
Research Article
Antioxidant and Biological Activities of Proteinaceous Extract from Algerian Glycine max Plant
Laboratoire de Chimie-Physique Moleculaire et Macromoleculaire, Departement de Genie des Procedes, Faculte de Technologie, Universite Saad Dahlab de Blida 1, B.P. 270, 09000 Route de Soumaa, Blida, Algeria
Saad Moulay
Laboratoire de Chimie-Physique Moleculaire et Macromoleculaire, Departement de Genie des Procedes, Faculte de Technologie, Universite Saad Dahlab de Blida 1, B.P. 270, 09000 Route de Soumaa, Blida, Algeria
Khaldoun Bachari
Centre de Recherche Scientifique et Technique en Analyses Physico-Chimiques, B.P. 384, RP 42004 Zone Industrielle Bou-Ismail, Tipaza, Algeria
Yamina Benmiri
Laboratoire de Biologie des Syst�mes Microbiens (LBSM), Ecole Normale Superieure (ENS), Kouba, Algeria