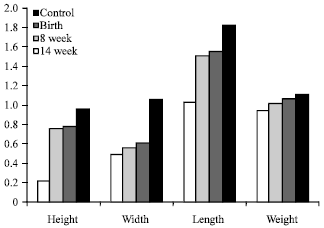
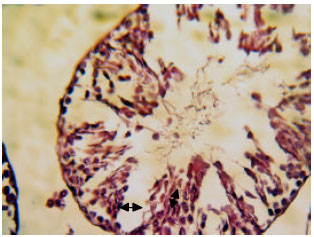
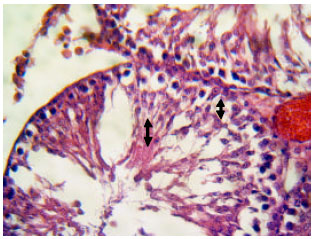
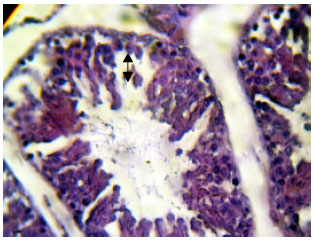
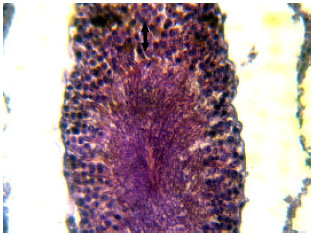
Research Article
Study of Spermatogenesis Fetal Testis Exposed Noise Stress During and after Natal Period in Rat
Physiology Research Center, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Masoud Hemadi
Fertility, Infertility and Perinatology Research Center, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Ghasem Saki
Physiology Research Center, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Alireza Sarkaki
Physiology Research Center, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran