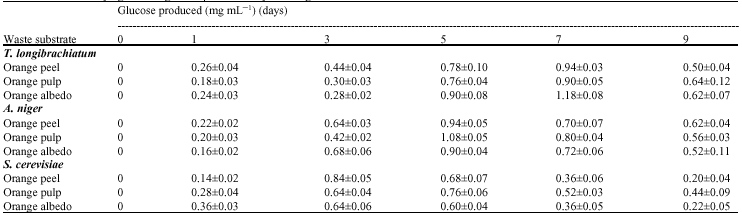
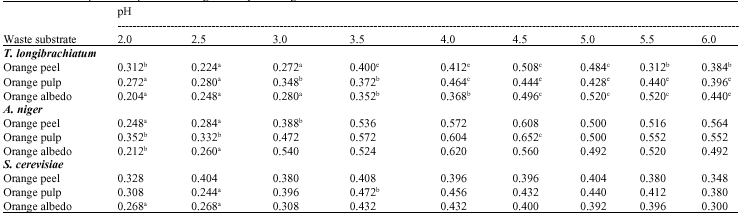
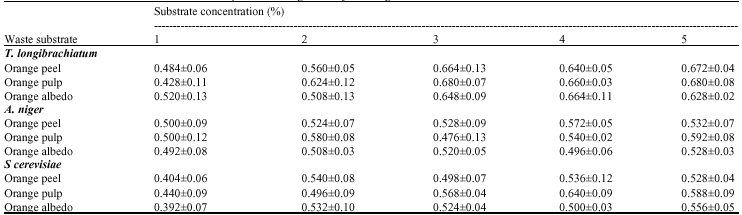
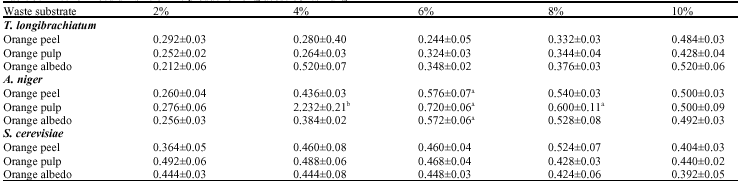
Research Article
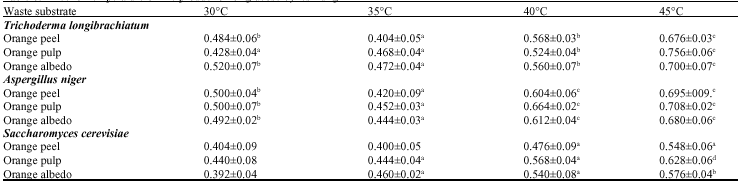
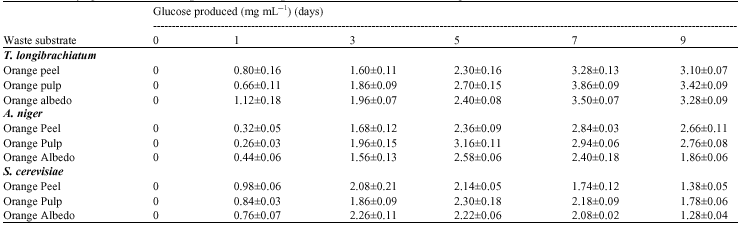
Cellulase Production by Trichoderma longi, Aspergillus niger and Saccharomyces cerevisae Cultured on Waste Materials from Orange
Department of Microbiology, University of Ilorin, Ilorin, Nigeria
O.P. Jilani
Department of Microbiology, University of Ilorin, Ilorin, Nigeria