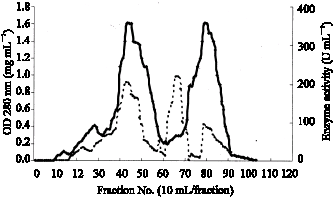
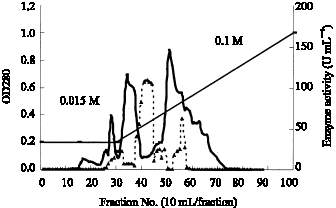
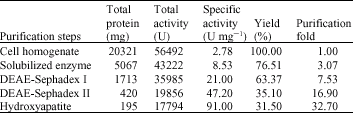
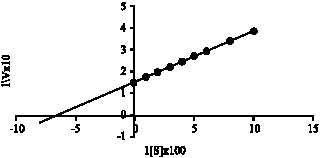Research Article
Purification and Characterization of Alcohol Dehydrogenase from Gluconobacter suboxydans
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran
A. Shabani
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran
M. Saifi-Abolhassan
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran
Sh. Sepehr
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran
M.R. Soudi
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran
S.Z. Mossavi-Nejad
Department of Biology, Faculty of Basic Science, Alzahra University, Tehran, Iran