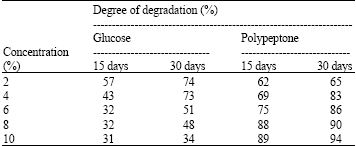
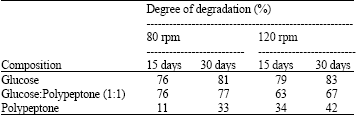
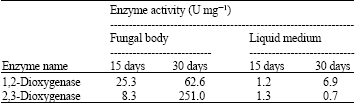
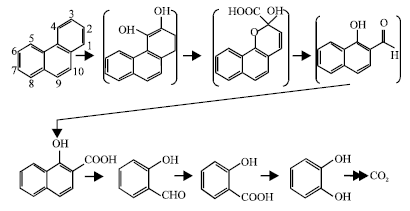
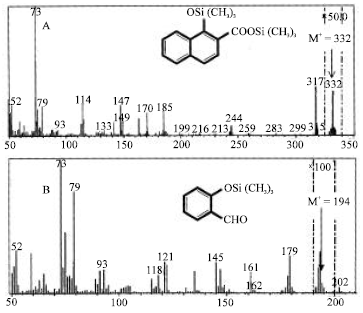
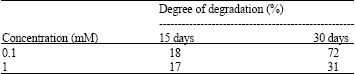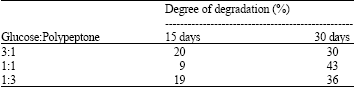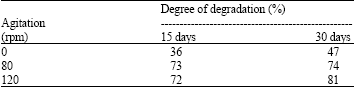Research Article
Biodegradation of Phenanthrene by Fungi Screened from Nature
Department of Applied Bioscience, Faculty of Agriculture, Ehime University 3-5-7 Tarumi, Matsuyama, Ehime, 790-8566 Japan
Sanro Tachibana
Department of Applied Bioscience, Faculty of Agriculture, Ehime University 3-5-7 Tarumi, Matsuyama, Ehime, 790-8566 Japan
Kazutaka Itoh
Department of Applied Bioscience, Faculty of Agriculture, Ehime University 3-5-7 Tarumi, Matsuyama, Ehime, 790-8566 Japan