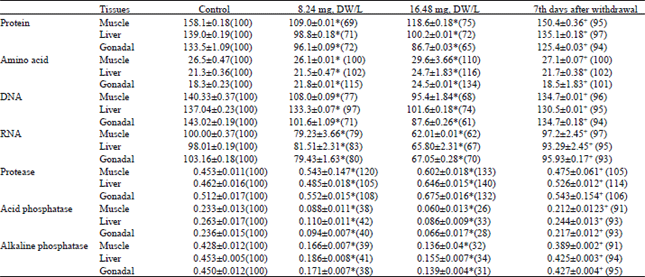
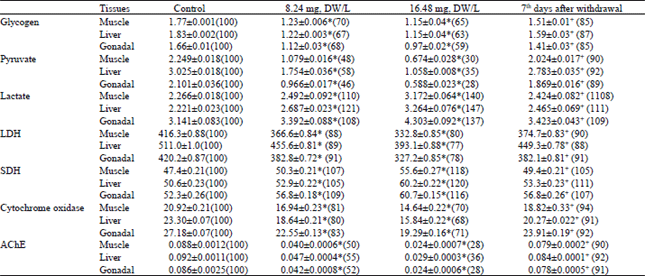
Research Article
Metabolic Changes in Freshwater Fish Channa punctatus Due to Stem-bark Extract of Croton tiglium
Natural Product Laboratory, Department of Zoology, D.D.U, Gorakhpur University, Gorakhpur- 273 009 (U.P.), India
Digvijay Singh
Natural Product Laboratory, Department of Zoology, D.D.U, Gorakhpur University, Gorakhpur- 273 009 (U.P.), India
S. K. Singh
Natural Product Laboratory, Department of Zoology, D.D.U, Gorakhpur University, Gorakhpur- 273 009 (U.P.), India
Ajay Singh
Natural Product Laboratory, Department of Zoology, D.D.U, Gorakhpur University, Gorakhpur- 273 009 (U.P.), India