Research Article
Cell Turnover in the Trigeminal Ganglion During Gangliogenesis and Aging Process and its Significance
Department of Anatomy, Faculty of Medicine and Health Sciences, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia
Two types of ganglionic neurones, the dark and the light types based on staining properties, have been documented in many vertebrates (Moses, 1967; Pineda et al., 1967; Carmel and Stein, 1969). Studies in the chick and rat (Gaik and Farbman 1973; Peach, 1972) concluded that these two types are genuinely different from each other in terms of density and distribution of cytoplasmic organelles. Differences in chemical constituents in these two types of neurones in the trigeminal ganglion have been reported (Kalina and Bubis, 1969; Silbermann and Finkelbrand, 1978). The significance of these two populations of neurones has been the subject of considerable speculations. Among these are the dual embryonic origin (epidermal-placodal and neural-crest origin) (Hamburger, 1961), difference in central and peripheral projections (Gobel, 1974) difference in sensory functions (Noden, 1980; Spassova, 1982; Sugimoto et al., 1989) etc. The accessibility of sensory ganglia from the earliest stages of development, and the refined techniques that have evolved, have lead to the greater understanding of the cellular and molecular development of sensory neurones (Altman and Bayer, 1982; Davies and Lumsden, 1990). In addition, the target-fields of certain developing sensory ganglia are well-defined and accessible to experimental studies enabling a direct investigation of regulatory influences of these target-fields on neural development (Davies and Lumsden, 1990; Lumsden and Davies, 1983). The aim of the present study was to carry out chronological quantitative light microscopic study of the histogenic organisation, distribution and behaviour of the trigeminal ganglion during the ontogeny of the chick. This is expected to elucidate the significance of the dark and light neurones. This study would form a basis for the future more elaborate clinically-oriented investigation.
The chicks Gallus gallus domesticus, White Leghorn breed were used in this study. Fertilised eggs were incubated. After every 24 hours, it was considered as Embryonic Day 1 (E1), Embryonic Day 2 (E2) etc till hatching (H). Embryos till hatching were removed carefully under ether anesthesia and aseptic conditions, and fixed in 10% formaldehyde solution at least for two weeks. Larger (older) embryos were cut transversely into suitable smaller pieces and labeled serially for future orientation. The tissues of older embryos (i.e., E15 and onwards till adult) were usually decalcified after fixation. After making paraffin blocks, serial sections of 8-10 microns were taken and stained by Cresyl Fast Violet for Nissl granules. Only a few selected stages which showed some remarkable changes are described in this work. These include E6, E8, E10, E13, E15, E18, chick on the day of hatching (H) and adult (A). In all, three animals in each group, with a total of twenty four animals were used. Ganglia of both sides were used for examination and every section was drawn and the cells were plotted in diagram with the help of a light microscope having a camera lucida attachment. The neurones were classified into dark and light types according to the difference in the intensity of cytoplasmic stain. Each of these types is again subdivided into various subclasses (size-categories).
Only those cells having a clear nucleus and a nucleolus were counted and measured with the help of an eye piece graticule. The following categories of cells were classified. Tiny (<5 microns), very small (6-10 microns), small (11-15 microns), medium sized (16-20 microns), big (21-25 microns), very big (26-30 microns), large (31-35 microns), very large (36-40 microns), giant (41-45 microns), gigantic (46-50 microns), gigantic giant (>51 microns). The size-categorisation of cells with a uniform difference of 5 microns was maintained just for the sake of convenience. However, this proved to be very useful in that, the behaviour, especially of the very-small cells, is very interesting on the day of hatching (uniformly) in all the ganglia studied. This explains that this stage of cellular-growth (very-small cells) is a critical stage during development, indicating a stage of active cellprocess-formation so as to establish functional connections with target tissues.
The ganglion showed great difference in different age groups of animals and in different areas of the same ganglion. These changes during successive embryonic days, on the day of hatching and in the adult situation were studied in greater detail (Table 1). The most striking changes are as follows. When the dark cells alone are present, they are represented just by their numbers; when they are mixed with light ones, D = dark neurones, and L= light neurones. The trigeminal ganglion could be recognised clearly on E6 while it had a rostra-caudal length of 0.376 mm and a volume of 0.0485 mm3. The ganglion had 73862 cells, all which were dark type. In all, 4923 (6.67%) were tiny cells, 24453 (33.11%) were very-small ones, 41267 (55.87%) were small ones, and 3219 (4.36%) were medium sized ones. On E8, the ganglion had a length of 0.600 mm, a volume of 0.1414 mm3 and had 259405 cells. Among these cells, 259327 (99.97%) were dark type and 78 (0.03%) were light ones. In all, 63347 (24.42%) were tiny cells, 168400 (64.92%) were very-small ones, 23973 (D = 23970 + L= 3) (9.24%) were small ones and 3685 (D= 3610 + L= 75) (1.42%) were medium sized ones. On E10, the ganglion had a length of 0.594 mm and a volume of 0.1909 mm3 and 101199 cells. Among these cells, 100603 (99.41 %) were dark type, and 596 (0.59%) were light ones. In all, 581 (0.57%) were tiny cells, 17067 (16.86%) were very-small ones, 59443 (D= 59095 + L= 348) (58.74%) were small ones, 24043 (D = 23804 + L= 239) (23.76%) were medium sized ones, 52 (0 = 45 + L= 7) (0.05%) were big ones and 13 (D = 11 + L= 2) (0.01%) were very-big ones. On E13, the ganglion had a length of 0.780 mm, a volume of 0.4641 mm3 and had 84493 cells. Among these cells, 52199 )61.78%) were dark type (and 32294 (38.22%) were light ones. In all, 510 (0.6%) were tiny cells, 27203 (32.2%) were very-small ones, 25841 (D = 10824 + L= 15017) (30.58%) were small ones, 22442 (D = 9856 + L= 12586) (26.56%) were medium sized ones, 7062 (D= 3131 + L= 3931) (8.36%) were big ones, 1140 (D = 503 + L= 637) (1.35%) were very-big ones, 283 (D = 163 + L= 120) (0.33%) were large ones, 4 (D= 3 + L= 1) were very-large ones and 8 (D = 6 + L= 2) were gigantic type. On E15, the ganglion had a length of 1.300 mm, a volume of 0.2004 mm3 and had 62441 cells. Among these cells, 34032 (54.5%) were dark type and 28409 (45.5%) cells were light ones. In all, 1004 (1.61%) were tiny ones, 19330 (30.96%) were very-small ones, 17374 (0= 5046 + L= 12328) (27.82%) were small ones, 16694 (D= 5742 + L= 10952) (26.74%) were medium sized ones, 5554 (0= 1833 + L= 3721) (8.89%) were big ones, 2479 (0= 1071 + L= 1408) (3.98%) were very big ones. On E18, the ganglion had a length of 1.300 mm, a volume of 0.7688 mm3 and had 306498 cells. Among these cells, 288252 (94.05%) were dark type and 18246 (5.95%) were light ones. In all, 113491 (37.03%) were tiny cells (highest, 106003 (34.59%) were very-small ones, 49614 (D= 43706 + L= 5908) (16.18%) were small ones, 32053 (D = 22305 + L= 9748) (10.46%) were medium sized ones, 4346 (D = 2154 + L= 2192) (1.42%) were big ones, 964 (D= 578 + L= 386) (0.31%) were very-big ones and 27 (D 15 + L= 12) were large ones. On the day of hatching, the ganglion had a length of 1.950 mm, a volume of 0.7873 mm3 and 58779 cells. Among these cells, 36116 (61.44%) were dark type and 22663 (38.56%) were light ones. In all, 781 (1.33%) were tiny cells, 17223 (D = 12288 + L= 4935) (29.3%) were very-small ones, 19557 (D= 11275 + L= 8282) (33.27%) were small ones, 14890 (D= 8138 + L= 6752) (25.33%) were medium sized ones, 3841 (D = 1856 + L= 1985) (6.53%) were big ones, 1733 (0= 1116 + L= 617) (2.95%) were very big ones, 679 (1)=. 605 + L= 74) (1.16%) were large ones, and 75 (D= 57 + L= 18) (0.13%) were very large ones. In the adult situation, the ganglion had a length of 3.750 mm, a volume of 2.7904 mm3 and 36826 cells. Among these cells, 29475 (80.04%) were dark type and 7351 (19.96%) were light ones. In all, 548 (1.49%) were tiny ones, 5512 (D = 3886 + L= 1626) (14.97%) were very-small ones, 3576 (D= 2539 + L= 1037) (9.71%) were small ones, 6167 (D= 4567 + L= 1600) (16.75%) were medium sized ones, 522 (D= 435 + L= 87) (1.42%) were big ones, 4381 (D= 3568 + L= 813) (11.9%) were very-big ones, 3996 (D= 3277 + L= 719) (10.85%) were large ones, 10191 (D= 8841 + L = 1350) (27.67%) were very large type, 10 (D= 7 + L= 3) (negligible) were giant cells, 1917 (D = 1803 + 114) (5.21%) were gigantic type of cells and 6 (D = 4 + L= 2) cells were of gigantic giant type.
The total number of cells observed in the ganglion on Embryonic Day 6 (E6) was 73862, and by E8 it had increased to 259405. On E10 there was a marked reduction in the number of cells to 101199. This had continued to reduce on -E13 with a total of 84493 cells. The reduction in the number of cells continued on E15 (with 62441 cells). Later a second dramatic increase followed on E18, and had reached its highest peak of 306498 cells. However, on the day of hatching, there was a great reduction in the number of cells to a total of 58779, followed by further reduction in the adult situation with 36826 cells. On day E8 when there was a high peak of dark cells (259327), a few light cells (78) also had appeared. The light cells continued to increase in number to reach its first high peak (32294) on E13, while the dark cells had declined to 52199. The light cells have started to decline on E18 to 18246. The dark cells had decreased in number to 34032 on E15 and then increased dramatically to 288252 on E18. The day of hatching marked the occurrence of a second high peak of light cells (with 22663 cells) while there is a decline of the dark cells (to 36116 cells). In the adult situation, the dark and light cells have declined in number to 29475 and 7351 cells respectively.
The present study shows that there were two periods of active proliferation and two periods of active degeneration which were indicated by the sharp fluctuations in the number of ganglionic neurones during the ontogeny of the chick. The periods of high proliferative activity are E8 and E18, and the periods of degeneration are between E10-E15 and later throughout the post-hatching period . This is similar to the observation (Carr and Simpson, 1978) that beyond E8 of incubation there is periods of degeneration of cells (E10-E15). The present results also agree with the observation of other investigators (Cowan and Wenger, 1967; Rogers and Cowan, 1973; Landmesser and Pilar, 1974; Hamburger, 1975; Kishida et al., 1982) showing that 40% or more of the neurones which are initially generated fail to survive.
The dark and light neurones, in varying numbers, were found dispersed at random within the ganglion as early as E8 and continued to be present throughout the rest of the ontogeny.
| Table 1: | Illustrates the distribution of cells in the trigeminal ganglion |
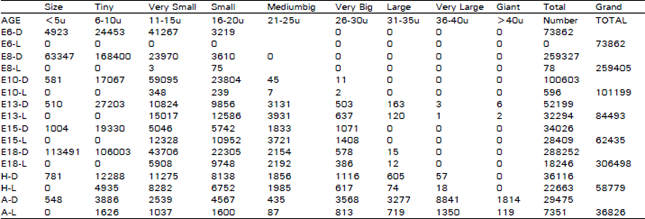 | |
| D = Dark Cells, L = Light Cells, E = Embryonic Age, H = Day of Hatching, A = Adult | |
However, this does not agree with the observations that such random distribution is found only in mature ganglia (Gaik and Farbman, 1973; Ciani et al., 1973; Noden, 1978) (i.e., as from 18th day of incubation to adult) in the chick. The coincidence of the occurrence of light cells combined with the decreased total cell-number indirectly but clearly suggests that these cells might represent inactive, dying, dead or degenerating cells. Probably, during this process, the light cells gradually become light-coloured by reducing the intensity of their cytoplasmic stain. This is similar to the view that the light cells might be the beginning stage of the process of inactivation and degeneration (Alvin, 1993). It is assumed that the occurrence of just 78 light cells on E8 might represent the beginning of cellular death and degeneration as early as E7 which in turn might suggest that the establishment of functional projection takes-place around E7. It is expected that the neurones which fail to establish functional connections will die and degenerate which should exhibit some abnormal characteristics (thereby changing to light-coloured cells on staining). It has been documented (Hamburger and Narayanan, 1969) in the trigeminal ganglion that by the 8th day of incubation, some of the cells have established peripheral and central connections as indicated by the presence of reflexogenic responses to tactile stimulus of the beak. It has also been suggested (Noden, 1980) that many trigeminal sensory cells have extensive projections by the end of the first week of incubation. The present results of structural evidence combined with the physiological observations of the above investigators might suggest that those cells which fail to establish proper central and peripheral connections might become inactive, lose their capacity and finally degenerate. It has been observed that an important function of cell-death is to regulate the number of the neurones to the requirements of their target-fields (Davies and Lumsden, 1990).
It is assumed from the behaviour of these dark and light cells that the period of accelerated degeneration corresponds to the period of active establishment of proper connections of the ganglion cells. This assumption is very similar to that of other investigators (Rubel et al., 1976). It has been shown (Yip and Johnson, 1984) that survival of mature neurones in the dorsal root ganglion is partially dependent on the availability of nerve growth factor (NGF) transported from the central nervous system to the peripheral ganglion. However, the recent studies have shown that agents other than NGF might guide neurites of NGF-responsive neurones during development (Lumsden and Davies, 1983, 1986; Lumsden, 1988). It is possible that even after establishment of projection fibbers to proper innervation fields, and having adequate NGF, cell death might occur due to the failure of these fibers to perform their functions due to other adverse factors. This is in accordance with the observations (Oppenheim, 1981; Korsching and Thoenen, 1985; Davies et al., 1987) that from 20-80% of neurones generated in sensory ganglia die shortly after they innervate their target-fields. As both central and peripheral target-fields play a role in regulating neural umbers (Davies and Lumsden, 1990), cellular death might occur due to an adverse factor on either side at any stage of their growth, and even after connections have been established. On the basis of the behavior of the neurones through the whole ontogeny, it is assumed that the dark cells represent a group of active cells whereas the light cells represent a group of either resting, inactive, dying or dead cells which have failed to establish functional projections.
The reduction in the total number of cells on E10 along with an increased number of light cells might indicate that the cellular death and degeneration are taking place faster within these few days. In connection with, it is also interesting to note that on E13 and E15, there is a reduced total number of cells and 38.22% and 45.5% of their cells (respectively) are light ones, indicating a greatly increased tendency of cellular inactivity and death during this period amongst nearly all categories of cells. There was a tendency of tremendously increased proliferative activity, growth and maturation of neurones on E18. Marked increase of all classes of cells was observed in addition to the appearance of some large ones. However, the light cells have reduced in number while the dark cells (most of them belonging to smaller categories) have increased. It is assumed that this influx of new group of smaller categories might represent a form of phagocytes, and therefore, suggestive of a greatly increased phagocytic activity at this stage, so that the inactive or dead cells (so-called light cells) are quickly being digested and removed from the vicinity of the ganglion, as has been suggested by earlier investigators (O'Connor and Wyttenbach, 1974; Pilar and Landmesser, 1976; Chu-Wang and Oppenheim, 1978). It is also suggested that only certain proportion of cells are active while other cells are resting, representing different stages of functional activity.
On the day of hatching, a greatly reduced number of cells, having a higher proportion (38.56%) of light cells is observed, suggesting a slow rate of phagocytosis while some others might represent resting stage as well. After hatching, there appears to be a continuous process of cellular growth and maturation as a result of functional maturity, as shown by a marked reduction of tiny cells and increased number of large categories. The loss of cells as from the day of hatching to adulthood might be due to functional reduction as a result of ageing process.
The present investigation shows that there are two periods of active proliferation and degeneration of the trigeminal ganglion neurones during the ontogeny of the chick. The appearance of light cells as early as E8 might be related to the establishment of functional connections around E7, i.e., by the end of 1st week of incubation. The light cells observed in the trigeminal ganglion during development might have been formed as a result of their failure to establish proper functional connections. Thus, this present assumption based on structural observation confirms the results of earlier physiological studies (Noden, 1980; Hamburger and Narayanan, 1969).
The author wishes to express his sincere and unlimited gratitude to Prrofessor. Dr. Dubbeldam JL, University of Leiden, Netherlands for his continued generosity, guidance, cooperation and support throughout this study. His continuous efforts and encouragement to make it as part of a thesis for the award of a DSc degree of Leiden University are greatly appreciated.