Research Article
Ophthalmic Pharmacology of N-acetylcarnosine Lubricant Eye Drops
Innovative Vision Products, Inc., 3511 Silverside Road, Suite 1 05, County of New Castle, Delaware USA 19810
| • | Presbyopia. |
| • | Open-angle primary glaucoma (in combination with beta-blockers). |
| • | Corneal disorders. |
| • | Computer vision syndrome. |
| • | Eyestrain |
| • | Ocular inflammation. |
| • | Blurred vision. |
| • | Dry eye syndrome. |
| • | Retinal diseases. |
| • | Vitreous opacities and lesions. |
| • | Complications of diabetes mellitus and other systemic diseases. |
| • | Contact lens difficulties, particularly with soft contact lenses. (Not only do the lubricants in the Can-C TM N-acetylcarnosine eye-drop help to make wearing contact lenses more comfortable, but n-acetylcarnosine is also able to reduce the build up of lactic acid in the eye, thus providing the lens to be left safely in the eye for longer). |
Innovative Vision Products, Inc. is a holder of the worldwide patent (including PCT International Publication Number WO 2004/028536 A1) for the application of N-acetylcarnosine for the treatment of ophthalmic disorders including cataracts.
Cataract, the opacification of the eye lens, is the leading cause of blindness worldwide (Vision Research, A National Plan 1999-2002) accounting for approximately 42% of all blindness (Vision Research, A National Plan 1999-2002). More than 17 million people are blind because of cataract and 28 000 new cases are reported daily worldwide (Kupfer et al., 1994). About 20.5 million Americans age 40 and older have cataracts, according to Prevent Blindness America's 2002 Vision Problems in the US report. By the year 2020, 30.1 million Americans in this same age group will have cataracts, as stated in a follow-up research study published in April 2004. Cataract, a clouding of the eye's lens, is the leading cause of low vision among Americans, contributing to half of all cases. Although most cataracts are related to aging, babies can be born with congenital cataracts. They can also develop in early childhood, in one or both eyes.
It is well established that a major factor involved in the development of cataract is oxidative insult (Spector, 2000; Dische and Zil, 1951; Spector and Roy, 1978; Garner and Spector, 1980; Augusteyn, 1981; Varma et al., 1979; 1982; 1984; Giblin et al., 1987; Harding, 1973; Zigler et al., 1989; Bhuyan and Bhuyan, 1984; Babizhayev et al., 1988). Oxidative stress associated with the formation of lipid peroxides is suggested to contribute to pathological processes in ageing and systemic diseases, such as diabetes, atherosclerosis, chronic renal failure, inflammation and retinal degenerative diseases known as statistically significant risk factors for cataract (Clayton et al., 1982; Harding and Crabbe, 1984; Costagliola et al., 1988; 1990; Simonelli et al., 1989; Babizhayev and Deyev, 1989). The observation that lipid peroxides are elevated in the lens membranes of some patients with cataract has drawn attention to these toxic oxidants (Babizhayev, 1996; 1989a; 1989b; 1989c; Bhuyan et al., 1986a; Borchman et al., 1989). Lipid peroxides can cause cataract, producing damage to both cell membrane and cytosol regions (Babizhayev and Deyev, 1989; Bhuyan et al., 1986; Borchman et al., 1989a; Borchman et al., 1989b; Bhuyan et al., 1986b; 1991; Mibu et al., 1994; Nishigori et al., 1986; Yagi et al., 1985).
Both lens development and cataract disease are affected by the environment of the respective tissues of the eye (Fig. 1). The aqueous humor contains about 4 μg mL-1 of high density lipoproteins, which evidently take part in the renewal of lipid compositions of the lens (Cenedella, 1984; 1983). The oxidative modification of lipoproteins in the presence of trace amounts of transition metals (copper or iron) is variously associated with lipid peroxidation (LPO), an increase in net negative charge, hydrolysis of phospholipid and fragmentation of apoprotein B and the oxidized lipid moieties of lipoprotein particles can be implicated to the lens toxicity triggering cataractogenesis (Babizhayev and Bozzo Costa, 1994).
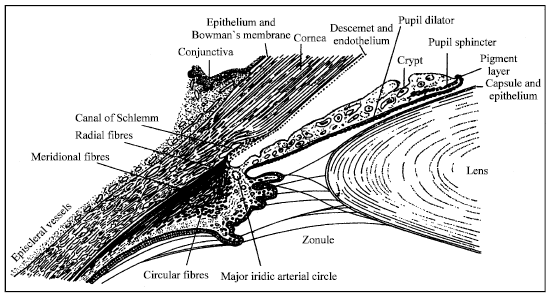 | |
| Fig. 1: | Scheme of the lens in the anterior chamber of the eye and its metabolism |
Previously published data suggest that L-carnosine (β-alanyl-L-histidine) has excellent potential to act as a natural antioxidant with hydroxyl-radical and singlet oxygen-scavenging and lipid peroxidase activities (Babizhayev, 1989b; Babizhayev et al., 1994; Dahl et al., 1988) ; thus it may be useful to prevent, or partially reverse, lens cataracts (Boldyrev et al., 1987; Babizhayev, 1989b). However, exogenous carnosine entering the organism intravenously, intraperitoneally, with food or topically to the eye, is not accumulated by the tissues but is excreted in the urine or destroyed by carnosinase, a dipeptidase present in blood plasma, liver, kidney and other tissues, except muscle and, probably, lens (Babizhayev, 1989b; Boldyrev et al., 1987; Babizhayev et al., 1989; Jay et al., 1990; Jackson et al., 1991; Lenney et al., 1985).
The N-acetyl derivatives of histidine, carnosine and anserine exist in the cardiac and skeletal mammalian muscles and the total concentration of these imidazoles may lie within the measured range of that of L-carnosine in skeletal muscle (i.e., ~ 10 mM) (O’Dowd et al., 1988). A knowledge of corneal and iris/ciliary body esterase activity, in particular, acetylesterase (EC 3.1.1.6) and in addition to esterase, the identified N-acetyltransferase activities (Anderson et al., 1980) prompted the development of a prodrug of L-carnosine in its ophthalmic application as antioxidant such as the chemically characterized N-acetylated form of the dipeptide (Babizhayev et al., 1996; 2000a). Due to relative hydrophobicity compared with L-carnosine, NAC might penetrate through the cornea gradually, thus maintaining longer the active therapeutic concentration of L-carnosine in aqueous humor of the treated eye (Babizhayev et al., 1996; 2000a). The clinical studies were validated in several issues to be a prospective evaluation of the lens opacities and visual function in cataractous patients who applied topically to the eye the physiologically acceptable solution of NAC (Babizhayev et al., 2002; 2001; 2000b).
Corneal changes resulting from contact lens wear are primarily due to hypoxia, but there is an effect due to a change in corneal pH that must be taken into account. The pH effect that accounts for some of the corneal complications accompanying contact lens wear are consistent with several studies which have demonstrated that intracellular pH can control many cellular functions including glycolysis, membrane ion secretion and mitotic activity (Rivera and Polse, 1996). It has been shown that stromal pH is lowered during contact lens wear (Bonanno and Polse, 1987) and it has been suggested that contact lens-lowered pH may cause changes in corneal structure and function. It appears that the lactic acid accumulation in combination with carbon dioxide build-up (Ang and Efron, 1990) is responsible for acidosis of the stroma and presumably the epithelium. Clinically-reduced pH has been cited as responsible for transient morphological endothelial changes, such as the bleb response (Holden et al., 1985) and endothelial pH changes have been exclusively associated with a build up of CO2 behind a contact lens (Giasson and Bonanno, 1995).
This overall study was conducted by the end of 20th century at the leading Ophthalmic and Fine Chemistry Technology Institutes associated with activities of Innovative Vision Products, Inc. Results of our study investigating the effects of N-acetylcarnosine and its bioactivated principle L-carnosine in in vitro and/or in vivo models suggest that these agents may have lens and cornea protective effects.
Patients
Aqueous humor and human lenses were obtained from patients undergoing intracapsular cryoextraction of senile or complicated cataracts and used as the test material. This project was approved by the ethic commission of Helmholtz Research Institute for Eye Diseases (No. 008/136/30). The control aqueous and transparent human lenses were obtained from eyes donated for corneal grafting supplied from a corneal transplant bank within 15 h post mortem. The mean age at surgery and the average donor age was 59 years ranging from 16 to 89 years. Both groups contained an almost equal number of males and females.
Aqueous Humor Sampling from Human Eyes
After retrobulbar and lid anesthesia the two vertical recti muscles were fixed. A stab incision was performed transcorneally 1 mm from the limbus in the temporal superior quadrant. Aqueous humor from human eyes (about 0.1-0.2 mL per eye) was aspirated from the anterior chamber of each eye with a 25-gauge needle connected to a tuberculin syringe immediately before surgery and shipped on wet ice under sterile conditions. Human aqueous humor was then briefly centrifuged at low speed to remove any cellular debris and the aliquots of fresh aqueous humor from each patient were immediately used for lipid extraction.
Lenses
The test and control material consisted of opaque human lenses at different stages of cataract or normal human lenses. The cataractous human lenses were obtained during operation of cataract surgery by intracapsular cryoextraction. Before surgery, all the lenses were examined by biomicroscopy and assigned to cataractous or transparent lenses in line with the clinical characteristics of clouding. The average age of the cataract patients and donors provided lenses was 65±9 years.
Rabbit transparent lenses were obtained from freshly enucleated eyes of the Chinchilla race animals. Normal mouse lenses were extracted from the eyeballs by the posterior approach from mice of the strain C57BL or hybrids F1 (CBA x C57BL) resistant to cataract formation. In all cases the integrity of the lens capsule was preserved. The lenses removed were briefly rinsed in Hanks’ medium for 20-30 sec and then immediately placed into a moist chamber. They were handled with the greatest care when being placed inside and being removed from their individual containers for measurements. The lenses were either used directly after the extraction procedure, or surgery, or at least 1-3 h were allowed to elapse between the dissection from the eye and the start of the measurement. This delay did not influence the results. To evaluate objectively the lens opacity by means of biomicroscopy and further photoregistration, an image of the lens was obtained on Leitz TV-analyser (Fig. 2). The values of the optic density in different lens zones were determined (Babizhayev et al., 1989). The development of quantitative morphometric criteria for evaluation of the lens opacities was presented by Babizhayev et al. (1989; 1992). Two characteristics were introduced for quantitative evaluation of lens opacity degree: (1) the opacity intensity characterized with optic density value and (2) the opacity extent defined as the opacity zone area. The OD values were set so as to be able to characterize the most dense, the less dense and transparent lens sections and to divide the lens image into zones of indicated OD values (so-called equidensities). Integral degree of the lens opacification (IDLC) is estimated as the ratio between the opacity zone area (which corresponds to maximum stages of the lens OD values) and the lens whole surface area, expressed as percentage. This measuring procedure was brief (5-10 min) and did not influence the further lens behaviour.
Electron-microscopic Study
For electron microscopy, whole lenses or pieces of lens tissue were fixed with 4% glutaraldehyde prepared in phosphate buffer (0.1 M, pH 7.2-7.4) and then with 1% OsO4 solution as described earlier (Babizhayev et al., 1988; Babizhayev and Deyev, 1987; Babizhayev and Brodskaya, 1991).
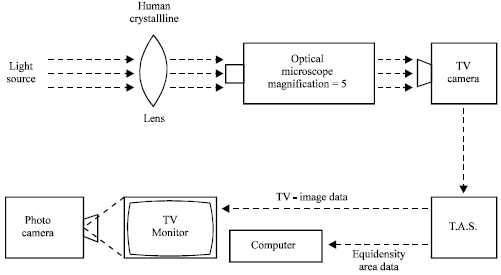 | |
| Fig. 2: | Image analysis procedure which makes it possible to obtain an image of the lens, to determine values of the optical density in different parts of the lens, to divide the lens surface into zones of defined density range, to measure the areas of equidensities and to evaluate the precise topography of every zone. |
Afterwards, the samples were washed with 50% ethyl alcohol. Lens dehydration was performed in alcohol of increasing concentration: 60, then in 2% uranylacetate on 70, 80, 96 and 100% alcohol. Furthermore, the samples were put in absolute acetone and were then kept in a mixture of acetone with epone resin (2:1); (1:2). The lenses kept in epone were maintained at 37°C (24 h) and then at 60°C (48 h). The sections for electron microscopy were performed perpendicularly to the surface in the medial lens zone area on an ‘LKB-200 11’ ultramicrotome. The contrasting stain was performed with lead nitrate by the Reynolds (1963) method. The samples were examined on a ‘Jem 100 B’ electron microscope.
Lipid Extraction and Quantification in the Aqueous Humor
Aliquots of fresh aqueous humor from each patient or animal were immediately extracted into 40 vol. of chloroform-methanol (2:1, v/v) with 4-methyl-2,6-ditert-butyl-phenol (BHT) antioxidant addition (0.5 mg/100 mL) during 10 min. The obtained extract was washed according to the modified Folch procedure and the chloroform layer was recovered and evaporated under argon (Babizhayev et al., 1988). Aliquots of the lipid residue were analyzed for total phospholipid phosphorus and fractioned to discriminate cholesterol, free fatty acids and the individual spots of the polar lipids as described previously (Babizhayev, 1996; Babizhayev and Bozzo-Costa, 1994).
Lipid Extraction from the Lens
Immediately after the lens material had been obtained, lipids were extracted from the lens by Folch method (Folch et al., 1957). The extraction was carried out by tissue homogenization in 20 vols. of chloroform/methanol mixture (2:1 by vol.) with BHT antioxidant addition (0.5 mg/100 mL) for 10 min. After filtration the sample obtained was put into a separating funnel for 5-8 h to stratify. Water was added in 7:1 ratio to promote the stratification. Temperature was maintained at 0°C for all the operations. After separation of the phases and removal of the aqueous-methanol layer, the lower chloroform fraction was evaporated. Phospholipid content was assessed by the results of organic phosphorus evaluation (Chen et al., 1956). Total lipid amount in the extract was determined gravimetrically, as well as by characteristic absorption in 206-210 nm area of the lipid sample after dissolution in 4 mL of methanol/heptane mixture (5:1 by vol.).
Determination of Lipid Peroxidation Products
Accumulation of the LPO primary molecular products was estimated spectrophotometrically from characteristic absorbents of diene conjugates in the UV-region at 232 nm characterizing the level of hydroperoxides of polyunsaturated fatty acids, as well as by LPO secondary molecular products absorbance at 274 nm, corresponding to the concentration of conjugated trienes and cetodienes (Bolland and Koch, 1945; Babizhayev et al., 1988) on HITACHI-557 spectrophotometer (Japan). The absoption spectra were recorded after the chloroform-methanol (2:1, v/v) extraction as described above and the dissolution of a dry lipid residue in 2.5 mL of methanol-heptane (5:1, v/v) mixture. This protocol removes any water-soluble secondary oxidation products, leaving them in the methanol-aqueous phase. The content of end molecular fluorescent LPO products was determined from the fluorescence intensity of the lipid extract at 365 nm excitation and 420-440 nm emission wavelengths (Chio and Tappel, 1969; Babizhayev et al., 1988), measured on a HITACHI-MPF-4 spectrofluorometer. The spectrofluorometer was calibrated at the beginning of every working day against a solution of quinine sulfate (1 μg mL-1 in 0.1 N H2SO4) standard, at 435 nm fluorescence emission and 365 nm excitation wavelengths.
Gas Chromatography of Halogen-substituted Derivatives of the Fatty Acids
Content of the polyunsaturated fatty acids in the lens is rather moderate, hence direct registration of their decrease in the course of LPO is difficult (Rosenfeld and Spector, 1982). However, to register directly an increase of oxiproducts in unsaturated fatty acids is of rather greater importance than to reveal a decrease in the acid content. In fact, it is the appearance of the fatty acid oxiderivatives as a LPO result in membrane lipid phase, that is well known, leads to their destruction (Kagan, 1988). In the applied gas-chromatography method we used not methyl esters of fatty acids but their halogen-substituted derivatives. By introduction of fluorine atom into fatty acid molecule we succeeded in selective labeling of its functional groups. Using this property, it is possible to determine the change of the number of oxigroups that gain in content in fatty acids in the course of LPO both under in vitro and in vivo conditions (Babizhayev et al., 1988). Electron-capture detector used in gas-chromatography of fluorine-substituted compounds was found to be more sensible than flame-ionization one: minimum detectable sample flow in substances with high affinity of the electron, such as fluorine-substituted compounds, for electron-capture detector, is of 10-11 g/s- that is why this method is the optimum one to measure even lower levels of oxiderivative fatty acids in tissue lipid extracts (Babizhayev et al., 1988).
For selective determination in the lens lipid fraction of the substances containing oxigroups, fluorine-substituted derivatives of the fatty acids were obtained and the proper assessment of oxiderivative fatty acids was provided in the lens lipid fraction derived from normal and cataractous lenses as described earlier (Babizhayev et al., 1988).
Pharmacokinetics of Topical N-acetylcarnosine Application
Formulations and Animals
Grey Chinchilla rabbits (male) aged 3-4 months weighing 2-3 kg were used. Animal experiments conformed to the guidelines of the ARVO Resolution on the Use of Animals in Research. Thirty min prior the ocular incision right eyes of rabbits were instilled with 80 μL of formulation A containing 1% N-acetylcarnosine (NAC) and the control right eyes of the separate rabbits were similarly instilled with their vehicles (placebo) solutions. Formulation A (Can-C TM) contained the following ingredients:
 |
*Or what is necessary to bring the solution up to around a pH of 6.3-6.5
Formulation A was presented in the final ophthalmic tubes (per volume of 2.5 mL) and in the moiety of the plastic bottles. Placebo (Formulation B) solution contained the same ingredients without NAC. The solution of NAC in the phosphate buffer was also administered in the right eyes of the separate rabbits. The following formulations were used:
Formulation C: Just NAC dissolved in sodium phosphates solution pH 6.3. This was the research 1% NAC formula with the preservative Thimerosol added at 0.004 g per 100 mL.
Formulation D (N-acetylcarnosine, 1% is added) Benzyl Alcohol dissolved in the sodium phosphate buffers, pH 6.3 and at the same dissolution rate.
Formulation E (N-acetylcarnosine, 1% is added). Phenyl Ethyl Alcohol dissolved in the sodium phosphate buffers, pH 6.3 and at the same dissolution rate.
Surgical Procedure
Topical anaesthesia of the rabbit eyes was performed after 25 min of instillation of the formula ophthalmic solutions with instillations of 4% lidocaine hydrochloride solution eye drops (three times with 1 drop at 1.5-2.0 min intervals). The eye drops of 4% lidocaine hydrochloride contain benzaltonium chloride preservative. When ocular anaesthesia was achieved, the lids were extended and fixed with the lid-holder and the ocular bulb was fixed by tweezers in the area of the inferior rectus muscle. A stab incision was performed transcorneally 1.0-2.0 mm from the limbus in the temporal superior quadrant. Aqueous humor (0.1-0.2 mL) was aspirated from the anterior chamber of a rabbit eye with 25-gauge needle connected to an insulin syringe and immediately introduced into an Eppendorf tube with addition of ethanol (0.2 mL), keeping the sample on ice before extraction.
Extraction of Imidazoles from Aqueous Humor
Extractions of imidazole-containing compounds from the aqueous humor aliquots were performed according to Babizhayev et al. (1996). Portions of aqueous humor were added to ethanol as above and thoroughly mixed (20°C, 15 min). Extracts were centrifuged (2000xg, 15 min) and the supernatants removed. Samples were frozen in the gradient of temperatures to -70°C and lyophilized using the apparatus JOAN (France). The lyophilized residue was dissolved in 1 mL of 0.1 M Na2 HPO4 (pH 2.1 adjusted with 85% phosphoric acid) and filtrated through the membrane filter with the dimensions of pores 0.22 μm directly prior the analysis.
Analytical HPLC
Reverse phase analytical HPLC was performed using a Breeze chromatography system (USA), detector Waters 2487 Dual λ Absorbance Detector, column (250 x 4.6 mm) Symmetry 300 C18 5 μm (Waters), loop 20 μL. The column was eluted isocratically at 30°C with the cited phosphate buffer 0.1 M Na2 HPO4 (pH 2.1) over 25 min at a flow rate of 1.0 mL/min. Eluates were monitored for absorbance at 210 nm. The standards of L-carnosine and N-acetylcarnosine were prepared by weighing of the dry material using the analytical balance Mettler Toledo (accuracy 0.00004) and were further dissolved in the phosphate buffer 0.1 M Na2 HPO4 (pH 2.1). The quantitative determination of L-carnosine and N-acetylcarnosine in the samples was undertaken using the technique of external standard according to the area of the peak and linear extrapolation. The standards of eye drops were prepared by dissolution of initial solutions of eye drops by 100 fold using the phosphate buffer 0.1 M Na2 HPO4 (pH 2.1). Statistical significance was evaluated by the unpaired Student’s t-test and p = 0.05 was taken as the upper limit of significance.
Peroxidation Reaction System
The techniques for phospholipid extraction, purification and preparation of liposomes (reverse-phase evaporation technique) have been described previously (Babizhayev, 1989a, b ,c; Babizhayev and Bozzo Costa, 1994 ).
Peroxidation of phosphatidylcholine (PC, derived from egg yolks) was initiated by adding 2.5 μM FeSO4 and 200 μM ascorbic acid to the suspension of liposomes (1 mg mL-1) in 0.1 M Tris-HCl buffer (pH 7.4). The incubations were performed at 37°C. The tested compounds, NAC and L-carnosine, were added at 10-20 mM concentration to the system of iron-ascorbate-induced liposome PC peroxidation. The kinetics of accumulation of lipid peroxidation (LPO) products in the oxidized liposomes were measured by reaction with thiobarbituric acid (TBA). The TBA assay was described previously (Babizhayev and Bozzo Costa, 1994). To determine conjugated dienes the lipid residue of the samples was partitioned through chloroform during the extraction procedure (Babizhayev and Bozzo Costa, 1994). Correlation of the extracted lipid concentrations to the measured phosphorus was done by means of characteristic absorption at 206-210 nm of the lipid sample (redissolved in 2-3 mL of methanol/heptane mixture 5:1, v/v). Accumulation of net diene conjugates corresponding to the level of lipid hydroperoxides was assessed from characteristic absorbance of diene conjugates at ~ 230 nm (μCD = 2.8x104 M-1 cm-1), in a Shimadzu UV-260 spectrophotometer (Japan) (Babizhayev and Bozzo Costa, 1994). Absorbance of the secondary LPO products at ~ 274 nm, corresponding to the concentration of conjugated trienes and ketodienes, was also measured spectrophotometrically from the lipid spectra (Babizhayev and Bozzo Costa, 1994).
Statistical significance was evaluated by the unpaired Student’s t-test and p = 0.01 was taken as the upper limit of significance.
Uptake of L-carnosine by the Lens
In a separate series of experiments, the kinetics of L-carnosine penetration into the isolated rabbit lenses were evaluated (five lenses studied). To assess the ability of the lens to accumulate L-carnosine, it was placed in 5 mM (or higher concentration) L-carnosine solution in Hanks’ salt medium (without bicarbonate, pH 7.4), containing 7 mM glucose (Babizhayev, 1989b) and incubated at room temperature (20°C). After 1 h, the extraction procedure was utilized (Babizhayev et al., 1989a) to isolate L-carnosine from the nonprotein fraction of the lens. The nonprotein fraction removed was stained with 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-chloride) reagent and then applied for TLC in aqueous ethanol (EtOH/H2O, 77:23). The concentration of L-carnosine in the sample was estimated spectrophotometrically from its characteristic absorbance at 420/600 nm in a Hitachi-557 double-beam spectrophotometer (Japan). Standard L-carnosine samples were used to evaluate the quantitative recording of the absorbance. TLC was applied earlier (Boldyrev et al., 1987) to evaluate the L-carnosine level in eye lenses.
Incubation of Lenses with Liposomes
To control effects of metal ions or potential OH• scavengers (such as glucose present at scavenging concentrations in standard culture media), the lens culture medium was composed of 10 mM Tris-HCl, 140 mM NaCl (pH 7.4), phosphate and glucose-free (Medium A) or glucose-containing medium nutritious to the lens comprising 50 mM Tris-HCl, 5.5 mM glucose, 4.0 mM KCl, 102.5 mM NaCl, 1.0 mM K2HPO4 (pH 7.2-7.4) (Medium B) (Babizhayev and Bozzo Costa, 1994). Both media were adjusted to 290-300 mOsmol with NaCl and equilibrated with 95% air and 5% CO2. When significant concentrations of oxygen scavengers or other agents were added, the change in osmolarity was compensated by decreasing the amount of NaCl used to adjust the media back to the required osmolarity following modification. The rabbit lenses were incubated in 3.0 mL of medium per lens at room temperature. The tests of lens integrity during incubations, to indicate that the lens is functioning normally, were performed as previously reported (Babizhayev et al., 1992). When necessary, the lens incubation media contained the liposome suspension (0.5 mg mL-1). Fixed aliquots of media (50-500 μL) were taken out at different times from the organ cultures of lenses for measurements of LPO products. Generally, the total incubation time was 3 h.
Apparent pK values of typical histidyl-imidazole in proteins and of imidazole groups of histidine-related compounds were measured according to Abe (2000).
Aqueous Humor
Lipid and phospholipid contents in aqueous humor samples obtained from human eyes were reported recently (Babizhayev and Bozzo Costa, 1994). Typical UV absorption spectra of lipids have their maximum in the 206-nm regions related to absorption of isolated double bonds of hydrocarbon phospholipid chains (Fig. 3). Lipid extract from normal aqueous humor exhibits a modest shoulder of absorption at about 230 nm and a certain absorption peak around 280 nm in the UV region. However, lipid extract of patients with cataract usually exhibits the stronger absorption shoulder at 230 nm, characteristic of conjugated double bonds in the fatty acids (primary LPO products, lipid hydroperoxides) and an absorption peak around 280 nm (characteristic of ketones, aldehydes, secondary molecular LPO products) (Fig. 3, upper curve). When the level of secondary products not removed into the water-soluble phase was not significantly increased in UV absorption spectra of lipid extracts of aqueous humor from the cataractous patients (Table 1), the distinct increase of the contents of the end fluorescent LPO products expressed in terms of arbitrary fluorescence units/μg phospholipids (PL) was about 2.4-fold and 4.2-fold higher correspondingly for immature or mature cataracts as compared to normal lenses (Table 1).
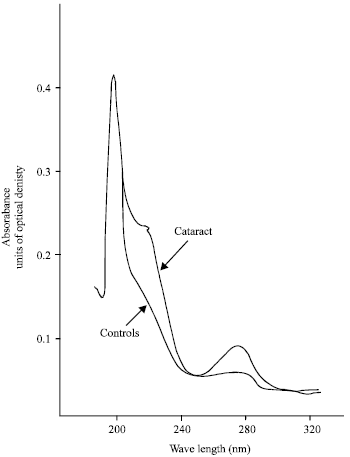 | |
| Fig. 3: | UV absorption spectra of lipid extracts (methanol-heptane 5:1, v/v) from the aqueous humor samples, obtained from human eyes: normal (control, lower curve) and mature cataract (upper curve) |
| Table 1: | Lipid peroxidation products in aqueous humor from the eyes of patients with cataract |
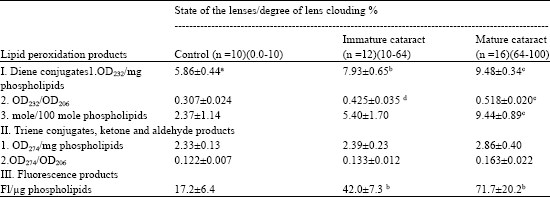 | |
| aMean±SD (n, number of the examined samples). Significant differences with control: bp<0.05, cp<0.001,d p<0.02 | |
In the samples of aqueous humor aspirated from donor eyes with normal lens, one in about 42 PL molecules contains a conjugated diene, while in case of immature cataract (IDLC 10-64%), the average amount of conjugated dienes reaches one in 19 aqueous PL molecules, whereas in mature cataract (IDLC 64-100%) this estimation averages one conjugated diene per 11 PL molecules. Net concentrations of primary (diene conjugates, phospholipid hydroperoxides) and end (fluorescent) LPO products were found to be increased in aqueous humor of the anterior chamber in cases of cataract irrespective of its genesis comparatively to donor (control) eyes and in line with the maturity stage measured by degree of clouding (Table 1).
Changes in Ultrastructural Organization of the Lens Fiber Membrane Integrity During the Development of Cataract
The electron microscopic observations have shown that, when the lens still preserves its transparency and protein aggregates cannot be detected in its tissue, the earliest changes in regular longitudinal lens fibers plasma membranes structure, did occur (Fig. 4a). They form typical knobs and Sockets (Fig. 4b). Consequently, the lens opacification is preceded by deterioration of the lenticular fiber plasma membrane ultrastructure. Following opacification progression the lens fibers become more irregular, their electron density increases, the membranes form numerous divertcula, twistings, interdigitations and fragments (Fig. 4c). Then further membrane fragmentation and an increase of the plasma membrane fragments’ curvature are observed. Figure 4d shows typical wave-like membrane structures forming multiple whorls around electron-dense centers. Such undulating structures differing by a number of different forms expand eccentrically from the lens fiber. Such formation has a string of pearls-like shape. At the mature stage of cataract, Fig. 4e, which is biochemically characterized by large high molecular aggregate formation in the human lens, ultrastructural characteristic of the membrane lesion is distinct with the lenticular fiber plasma membrane twisted fragments becoming the central mass of amorphous electron-grey debris and globules of different size (100-900 nm) and electron-dense granular contents. At the same time, fiber and membrane structures could no longer be identified and fibers appeared to have been converted to extensive masses of globular structures. First, smaller globules (100-200 nm) filled with granular material (probably of protein nature) of about the same density as the human lens fiber cytoplasm, form and then intermediate size globules (220-500 nm) containing aggregates of high density and dispersity appear. The loss of lenticular fiber-free membranes is probably connected with the mentioned membrane involvement in the protein aggregation. The larger globules (> = 600 nm) seem to develop from the small via the intermediate globules (Fig. 4f). At the latter stages of the human lens opacification, the globules become more and more coiled and aggregated, intermediate filaments disappear. At this stage, the lens matter is filled mainly with protein aggregates. Significant scatter of light accompanying the protein aggregate formation depends upon the incident wavelength of light in relation to the size and the concentration of globules.
Accumulation of Lipid Peroxidation Products in Human Lenses During Cataract Formation
The results of determination of the LPO different molecular products revealed in the lipid extracts from human lenses are presented in Table 2, Fig. 5a and b. It is evident that the content of the hydroperoxides having conjugated double bonds and determined by characteristic maximum in UV spectrum at 230 nm, increases at the initial stages of the opacification up to almost mature stage of cataract. However, at the further stages of mature and hypermature cataract the LPO primary molecular products level drops a little. At the same time, determination of the content of end molecular fluorescent LPO products-Schiff bases determined by fluorescence intensity of lipid extract at 430 nm (fluorescence excitation, 365 nm) revealed an increase monotonously along with the cataract development (Table 2, Fig. 5b). Figure 5a shows that the maximum of accumulation of the LPO primary molecular products in cataract lens attains its maximum at the stage of 55-64% opacification degree.
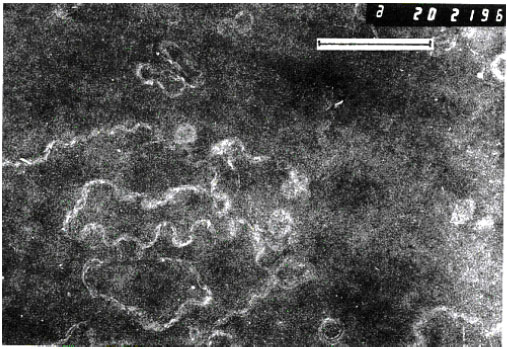 | |
| Fig. 4a: | Electron micrograph of superficial posterior cortex of the cataractous human lens showing apparently normal lens fibres exhibiting a moderate degree of interdigitation, lying outside the cataract zone. Bar, 1μm |
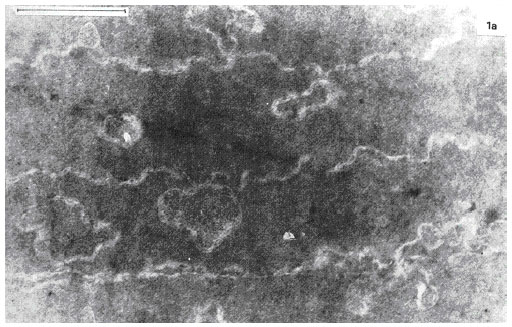 | |
| Fig. 4b: | Electron microphotograph of the midzonal area of the normal human lens. Bar, 1 μm. Changes in regular longitudinal lens fibers plasma membranes structure form typical knobs and Sockets |
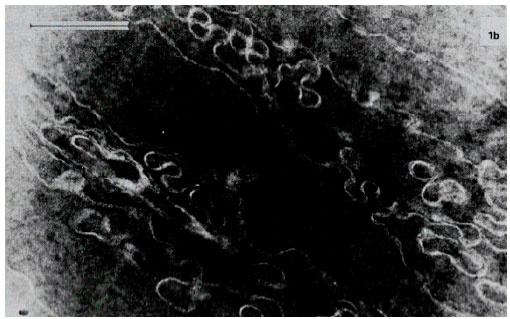 | |
| Fig. 4c: | Modification of the plasma membranes of the lens fiber cells by the lens opacification. Electron microphotograph of the midzonal area of the human lens. Immature cataract. Bar, 1 μm |
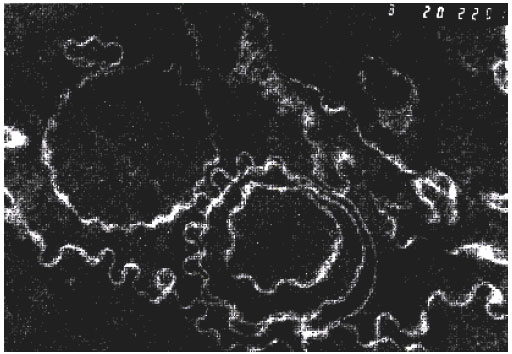 | |
| Fig. 4d: | Modification of the plasma membranes of the lens fiber cells by the lens opacification. Electron microphotograph of the midzonal area of the human lens. Immature cataract. 1 μm scale as in Fig. 4c. Figure 4d shows typical wave-like membranae structures forming multiple whorls around electron-dense centers |
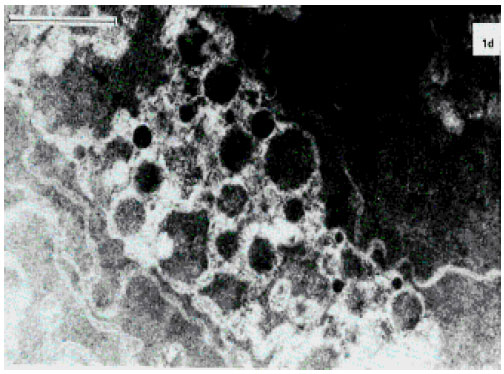 | |
| Fig. 4e: | Electron microphotograph of the midzonal area of the human lens. Bar, 1 μm- Nuclear cataract |
 | |
| Fig. 4f: | Electron microphotograph of the midzonal area of the human lens. Bar, 1 μm- Mature cataract |
| Table 2: | Content of lipid peroxidation products in human lenses. The data are presented as mean±SD |
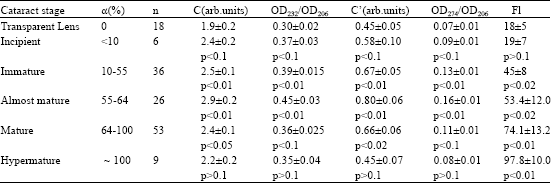 | |
| Notes: α, the lens opacity degree, C, the diene conjugates concentration, C~ OD232/CL, OD232, OD274, OD 206 optical density at 232 nm, 274 nm, 206 nm, respectively; CL, lipid content, mg/ml; C’, the triene conjugates (cetodienes) concentration, C’~ OD274/CL; Fl, fluorescence intensity of lipid extract in relative units ; n, the number of examined lenses; p<0.05, reliable values (compared with transparent lenses) | |
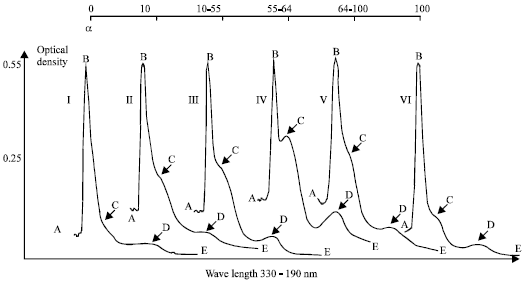 | |
| Fig. 5a: | The dynamics of the accumulation of the primary molecular lipid peroxidation products during the opacification of the human lens. α-the lens opacity degree, %; I-VI- human cataract stages; A-190 nm, B- 206 nm, C- 230 nm (diene conjugates maximum), D- 274 nm (triene conjugates maximum), E- 330 nm |
At the same time, the concentration of the end fluorescent LPO products in the lens correlates strongly with the integral lens opacity degree (r = + 0.956, p<0.01). Meanwhile, an important regularity was revealed: accumulation of the LPO products in the lens depends on the cataract development stage, but does not depend on its kind, allowing to presume a universal role of the LPO process in the lens opacification.
The Study of Halogen-substituted Derivatives of Fatty Acids in the Human Lens
Typical chromatograms of lipid fraction from transparent and cataractous (mature cataract) human lenses are shown in Fig. 6a and b. Although in mature cataract, on gas-chromatographic profile of the lens lipid sample, no principally new peaks are found to appear, the intensity of peaks markedly differs from the norm as quantitative ratio.
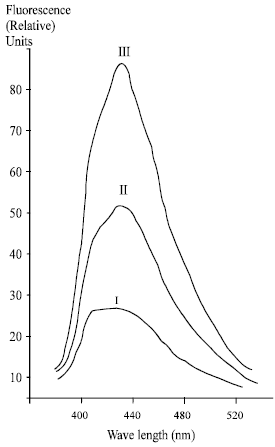 | |
| Fig. 5b: | Characteristic fluorescence spectra of the lipid extracts from the human lenses by excitation wavelength of 365 nm; I-norm; II- almost mature cataract; III- mature cataract |
| Table 3: | Analysis of fluorinated derivatives of fatty acids in transparent and cataractous human lenses |
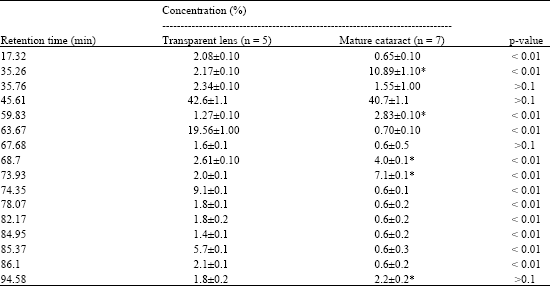 | |
| Notes: 1) Oxyproducts accumulation ratio in comparison with transparent lens is of 17.2%. 2) * Chromatographic peaks characterizing fatty acid oxyproducts accumulation | |
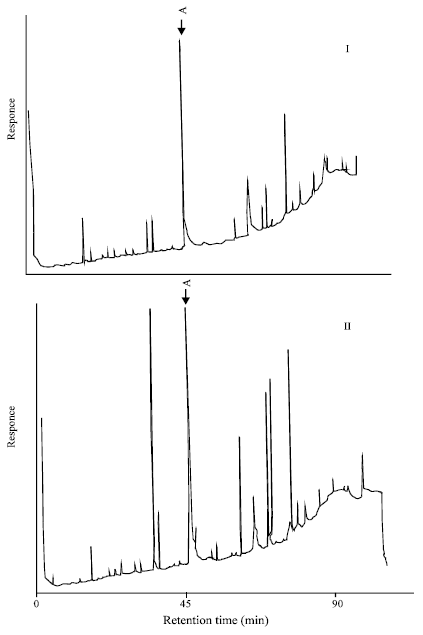 | |
| Fig. 6A and 6B: | Gas-chromatograms of the lipid fraction from transparent (I) and cataractous (mature cataract) (II) lenses after the fluorination. A- 18:1. X-axis: Retention time, min |
“A” peak corresponds to the fatty acid 18:1. As this method allows the determination of both fatty acids and their oxidized metabolites, in the absence of a complete set of their standards, no identification of all the chromatographic peaks was performed in the present study. However, a reliable increase in the intensity of the peaks whose retention time does not correspond to the fatty acids non-oxidized standards (Table 3), most probably reflects the fact of the increase in content of oxiderivative polyunsaturated fatty acids in cataract.
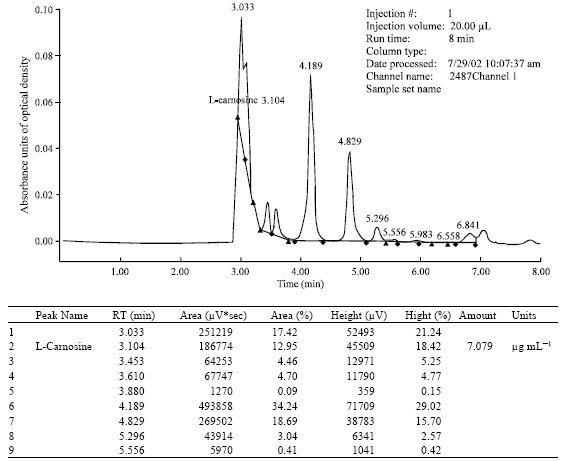 | |
| Fig. 7: | HPLC of extract of aqueous humor aspirated 30 min after the instillation of ophthalmic formulation with 1% N-acetylcarnosine and lubricants into the rabbit eye. N-acetylcarnosine RT is 5.83 min (amount: 0.153 μg mL-1); RT: Retention time |
N-acetylcarnosine as a Time Release (Carrier) Version of L-carnosine in Ophthalmic Applications with Carboxymethylcellulose
An important chemical difference between carnosine and N-acetylcarnosine is that carnosine is relatively insoluble in lipids (fats and fatty compounds), whereas N-acetylcarnosine is relatively soluble in lipids (as well as in water). This means that N-acetylcarnosine may pass through the lipid membranes of the corneal tissue and cellular structures more easily than carnosine and may thereby gain the access more readily to the intraocular aqueous humor. N-acetylcarnosine can gradually release carnosine which then exerts its beneficial effects (Babizhayev et al., 1996). In the present section of the study, we considered whether NAC acts in the ophthalmic formulation with lubricants (including carboxymethylcellulose) and preservatives when topically administered to the eye as a time release carrier (prodrug) of L-carnosine. The HPLC pattern of an extract of the aqueous humor obtained 30 min after instillation to the rabbit eye of ophthalmic formulation containing 1% NAC, lubricants carboxymethylcellulose, glycerine and preservative benzyl alcohol in the borate buffer confirms that the peak characteristic of L-carnosine has a concentration and a retention time (3.1 min) clearly distinct from N-acetylcarnosine (6.0 min) and from the dead time of the column (Fig. 7). This identified peak of L-carnosine was quantified and the data processor integrated that virtually all N-acetylcarnosine after the overall extraction efficiency is converted into the L-carnosine compound with a retention time of 3.1 min (Fig. 7). The data on the L-carnosine-related product were blanked with the control placebo data applied to the matched eyes of the control rabbits. The mean ratio of L-carnosine (C)/(NAC) relevant to the L-carnosine release in the aqueous humor 30 min after instillation of Formulation A with 1% N-acetylcarnosine into the rabbit eye corresponded to C/NAC = 6.64±0.06 (n = 8, where n= number of the examined treated rabbit eyes; only right eyes were treated). In the control placebo formulation-treated eyes the same indices could not be quantified at statistically significant rate. Concentrations of imidazole products in the aqueous humor corresponded to those of intact rabbit eyes and refer to baseline values of L-carnosine 0.19±0.10 μg mL-1 related products variously detected in extracts from normal animals. When control formulations contained only 1% N-acetylcarnosine dissolved in a buffered solution or contained 1% N-acetylcarnosine and added preservative Thimerosol, Benzyl Alcohol or Phenyl Ethyl Alcohol dissolved in a buffered solution (Formulations C-E, see Section 2.8.1), the C/NAC ratio corresponded to 1.99±0.05 (n = 7), 1.94±0.12 (n = 5), 1.98±0.05 (n = 5), 1.95±0.05 (n = 5), relatively. Our data demonstrate that topical administration of pure L-carnosine (1% solution) to the rabbit eye (instillation, subconjunctival injection) does not lead to accumulation of this natural compound in the aqueous humor over 30 min in concentration exceeding that in the placebo-treated matched eyes and its effective concentration is exhausted more rapidly (Babizhayev et al., 1996, 2000). In another aspect, the data demonstrate a method for the prevention or treatment of an eye disease, comprising topically applying toa mammal in need of said treatment an aqueous ophthalmic composition comprising N-acetylcarnosine or a pharmacologically acceptable salt of N-acetylcarnosine, in combination with a lubricant (cellulose compound) in an amount effective to increase intraocular absorption of L-carnosine into the aqueous humor.
Antioxidant Activity of N-acetylcarnosine Versus L-carnosine in the Liposome Peroxidation System
The comparative antioxidant activity of NAC and L-carnosine was assessed in the liposome peroxidation system catalyzed by Fe2+ + ascorbate (Fig. 8). The accumulation kinetics of molecular LPO products such as MDA and liposomal conjugated dienes and trienes are shown in Fig. 8 (a, b and c). The results demonstrate that the LPO reactions in the model system of lipid membranes are markedly inhibited by L-carnosine. The effective concentrations of L-carnosine are 10 and 20 mM. Data on the biological effectiveness of L-carnosine as antioxidant preventing PC liposomal or linoleic acid peroxidation in physiological concentrations ranges of 5-25 mM have already been published ( Babizhayev et al., 1994; Babizhayev, 1989b; Babizhayev and Bozzo Costa, 1994). Figure 8a shows that the level of TBA reactive substances (TBARS) reached at 5-min incubation decreases in the presence of L-carnosine (10 or 20 mM) at 10 min and at later time points (20 mM), which must be due to a loss of existing TBARS or peroxide precursors of MDA and not due to a decreased formation of peroxide compounds. The ability of the histidine-containing compound NAC to inhibit the (Fe2+ + ascorbate)-induced oxidation of PC liposomes was compared with that of equimolar concentrations of L-carnosine. The antioxidant activity of 10 and 20 mM NAC corresponded to 38 and 55% inhibition of LPO for the two concentrations after 60 min incubation. NAC exhibited less antioxidant protection than L-carnosine, corresponding to 60 and 87% of the equimolar (10 or 20 mM) L-carnosine inhibition percentage. However, since NAC can act as a time release version metabolized into L-carnosine during its cross through the cornea to the aqueous humor (but not oral use), the antioxidant activity of NAC in vivo application is significantly increased. Once released from NAC, L-carnosine in the aqueous humor might act against peroxidation of the lens during its target pharmaceutical use.
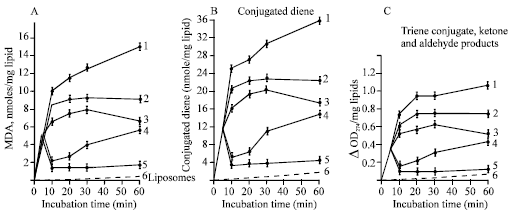 | |
| Fig. 8: | Accumulation of lipid peroxidation products (TBARS, measured as MDA) (A), diene conjugates (B), triene conjugates and ketone and aldehyde products (274 nm absorbing material) (C)) in liposomes (1 mg mL-1) incubated for 60 min alone (6, dotted line) and with addition of the peroxidation-inducing system of Fe2+ + ascorbate (1). Antioxidants N-acetylcarnosine (NAC) (10 or 20 mM) (2, 3) or L-carnosine (10 or 20 mM) (4, 5) were added at the fifth minute of the incubation period to the system containing the peroxidation inducers. Samples were taken at zero time and at time intervals indicated in the figures and were used immediately for measurement of TBARS. A similar amount of sample was partitioned through chloroform and used for detection of conjugated dienes and trienes dissolved in 2-3 mL of methanol-heptane mixture (5:1 v/v) |
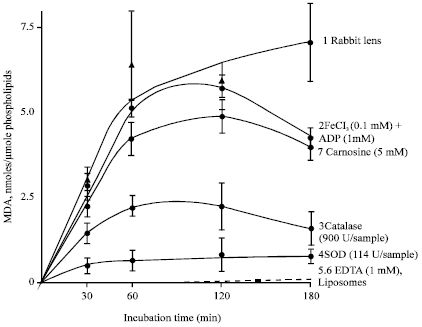 | |
| Fig. 9: | Effect of various oxygen radical scavengers on lipid peroxide formation in liposomes added to the incubation medium of the normal rabbit lens |
The biological effectiveness of L-carnosine as specific scavenger for activated oxygen species was assessed in the crystalline lens- induced LPO system. Transparent rabbit lenses were incubated in the various media containing 0.5 mg mL-1 liposome suspension as the oxidation substrate and the kinetics of the LPO reaction were estimated by measuring MDA, liposomal conjugated dienes and trienes making appropriate corrections for liposome autooxidation (Babizhayev and Bozzo Costa, 1994). In a background study in the absence of the lens, virtually no oxidation of liposomes took place during 180 min (Fig. 9, curve 6). In the presence of the lens, however, a marked increase in concentration of different molecular LPO products was demonstrated for the appropriate time of incubation both in glucose-free (A) or glucose-containing (B) culture media (Babizhayev and Bozzo Costa, 1994; Fig. 9, curve 1). The level of MDA accumulation after incubation of rabbit lenses for 3 h was 3.5- and 5.3-fold higher than of normal human or mouse lenses. The larger normal lenses (rabbit or human) have more epithelial cells rich in reduced glutathione (GSH), which are primarily metabolically active and these lenses can generate the active oxygen species and lipid peroxides more rapidly than cataractous lenses, with their exhausted pool of reductants, or tiny mouse lenses (Babizhayev and Bozzo Costa, 1994). In some cases a small decrease in the liposomal MDA concentration after incubation for 2 h was observed. This may be connected with MDA utilization by the lens itself (interaction of MDA with amino group, or its lowering by lenticular aldehyde dehydrogenase (Babizhayev and Bozzo Costa, 1994). A considerable reduction in the accumulation rate of the liposomal LPO products was found after the addition of catalase (900 U/sample) (Fig. 9, curve 3). This suggests a role of H2O2 in promotion of LPO by the lens. Addition of superoxide dismutase (SOD; 114 U/sample) to the incubation medium of the lens led to a marked reduction of the liposomal MDA level (Fig. 9, curve 4), suggesting that the lens could generate O2-C in the surrounding medium. Addition of the ADP-Fe complex to the incubation medium of the lens decreased accumulation of TBA-reactive material in liposomes by 33-50%, indicating decomposition of the accumulated TBARS. Almost total inhibition of TBA-reactivity in the liposomes occurred after the addition of the chelating agent 1 mM EDTA which eliminates free and accessible metal ions from the peroxidizing system (Fig. 9, curve 5). L-Carnosine has been shown to act as a good scavenger of the lipid peroxyl (LOO•) and hydroxyl (OH•) radicals (Babizhayev et al., 1994; Aruoma et al., 1989). The presence of 5 mM L-carnosine in the rabbit lens/liposome-containing medium decreased the TBA-reactivity by approximately 25% at 2 h incubation (Fig. 9, curve 7).
Study of L-carnosine Uptake by the Lens
To study the kinetics of L-carnosine penetration into the lens, the isolated rabbit lens was placed and incubated in a medium containing L-carnosine (5-15 mM). Incubation of lens preparations with histidine dipeptide led to its accumulation in the lens tissue (Fig. 10). It can be concluded that L-carnosine penetrates the barrier of the lens capsule when present in the aqueous humor at effective concentrations. The presence of L-carnosine in transparent crystalline lenses was detected and its concentration in this case was about 25 μM in normal human lenses and 0.89±0.1 mM (n = 4), rabbit lenses (Boldyrev et al., 1987; Jay et al., 1990). At different stages of cataract development, the level of L-carnosine fell, reaching about 5 μM in ripe human cataracts (Boldyrev et al., 1987). Thus, L-carnosine that finds its way into the aqueous humor can accumulate in the lens tissue for a reasonable period of time.
Tissue and Intracellular Buffering of N-acetylcarnosine (L-carnosine) in the Cornea
N-acetylcarnosine/L- carnosine provide intracellular buffering to stimulate anaerobic energy formation. The structural integrity of the cornea is maintained by an active transport system, which depends on metabolism (Fig. 11). Corneal acidosis during contact lens wear is significant under a variety of wearing conditions and thus, must be considered when evaluating the morphological or functional changes that occur following long-term contact lens wear.
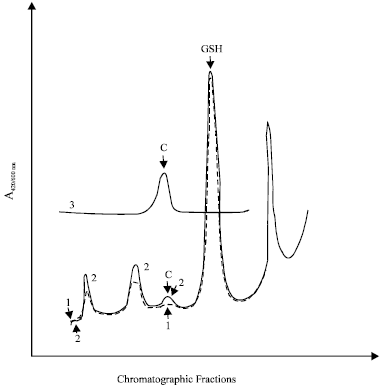 | |
| Fig. 10: | Absorbances of the extracted rabbit lens components after TLC separation. Curve 1 (broken line), extract of the control lens incubated for 60 min in Hank’s medium. Curve 2, extract of the lens incubated for 60 min in a medium containing L-carnosine (5 mM). Curve 3, a standard preparation containing L-carnosine (1 mM) in Hanks’ medium. C, L-carnosine maximum; GSH, reduced glutathione |
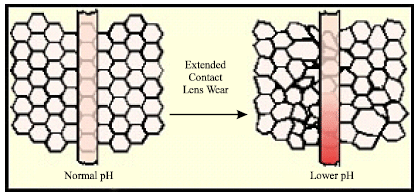 | |
| Fig. 11: | Contact lens wear can cause corneal complications such as ulcerative keratitis, neovascularization, epithelial microcysts, endothelial polymegethism, reduced mitotic activity, altered wound healing and reduced corneal function. Some of these complications can lead to permanent visual impairment. The prevention of these complications requires an understanding of the fundamental mechanisms responsible for changes in corneal structure and function. Hypoxic or extended wear (sleeping with the lenses on, referred to as closed-eye contact lens wear) can result in corneal acidosis, which alters corneal structure and function |
| Table 4: | Apparent pK values of imidazole groups (Abe, 2000) |
 | |
| Note: pK measurements were performed at temperatures specified in parentheses | |
The pH of corneal epithelium, stoma epithelium and aqueous humor decreased significantly with lenses up to Dk/L = 100. Hypoxia had large effects on the corneal epithelium and stroma, but had no effect on the endothelium or aqueous. Conversely, carbon dioxide accumulation caused significant acidosis in all compartments measured (Bonanno, 1996). The intracellular non-bicarbonate buffering of corneal tissue is associated with the imidazole group which exists in histidine residues of constituent corneal tissues, tear and aqueous humor proteins, in free L-histidine and in histidine-containing dipeptide(s) such as topically applied N-acetylcarnosine lubricant eye drops (time release version of L-carnosine). Because the pK values of these imidazole groups are close to pHi, one of the two nitrogens of the imidazole ring can be protonated in the physiological range of pH. Thus, imidazole groups are utilized as potent proton buffering constituents in corneal tissues. The regulatory process keeping pHi close to the pK values of imidazole groups is called alphastat regulation. Its role is to maintain α-imidazole relatively constant (α-imidazole being defined as non-protonated imidazole/(non protonated imidazole + protonated imidazole)). Typical αimid is conserved at a value of about 0.55 in intracellular fluid (Abe, 2000). Inorganic orthophosphate also serves as a typical inorganic buffer component in addition to imidazole compounds (Table 4). Acetylation of L-carnosine, L-histidine or carnosine derivatives does not change the capacity of imidazole compound to bind the protons in the region of neutral and weak acid pH values (Boldyrev, 1998). From the Introduction and data presented and discussed in this article, carnosine appears to be an efficient intracellular pH buffer, heavy metal chelator, potent anti-glycating agent and regulator of many specific receptors and enzymes. Our clinical observations demonstrate that the feeling of 'dry eye' is one of the most frequently reported symptoms by contact lens wearers. Epithelial lactate production increases during periods of corneal hypoxia, which causes an osmotic imbalance leading to increased stromal hydration. Further findings obtained at our Laboratories indicate that extended wear of hydrogel contact lenses reduces stromal keratocyte density. The mechanisms for this alteration may include hypoxic, cytokine-mediated or mechanical effects. The role of released carnosine in corneal disorders indicate on relation to tissue buffering, osmoregulation and antioxidation. These features make the developed N-acetylcarnosine ophthalmic prodrug lubricant eye drops useful to treat the contact-lens-induced stromal acidosis that cause corneal complications. Enhancement of the stability of the pre-lens tear film arising from the instillation of another re-wetting solutions was found to be transient. It was concluded that these lubricants are unlikely to provide a physical basis for prolonged symptomatic relief. The information obtained from our data can further an understanding of the etiology of corneal complications resulting from contact lens wear and will also improve present contact lens treatment strategies to help eliminate those lens-wearing conditions where stromal acidosis is likely to place the patient at risk.
The presented morphological and biochemical studies of human cataracts demonstrate that damage to the lens fiber membrane is involved in early stages of the development of the opacity. In the course of ageing, in which however, the lens transparency is still preserved, we may note increasing changes in regular order of the lens fiber cell packing. We suppose that this is related with detergent action of the phospholipid oxidation products accumulated in lenticular fiber membranes, which impair lipid-lipid and protein-lipid interactions. In this way changes are induced in membrane geometry (Babizhayev et al., 1988). Phospholipid molecules modified with oxygen, incorporating themselves into the lipid bilayer, change its geometry, diminishing the area of its hydrophobic portion, increasing its surface curvature owing to shortened radius of a membrane particle. This effect is obviously the basis of the membrane fragmentation and smaller vesicle formation in cataract. It is also possible that the change of lenticular membrane bilayer geometry can occur under the influence of phospholipid formation with Ca2+ ion complexes. An intrusion of proteins from the membrane surface to the water surroundings occurs as a result of impairment of hydrophobic-hydrophylic balance of the lipid bilayer. Membrane integrity is disrupted during the development of cataract. The mature cataract showed various types of cell disruption in the perimeter but not in the core of the nucleus. In line with another published study of age-related or senile human nuclear cataracts where morphological changes were examined using electron microscopy of thin section. Costello et al. (1992) present data suggest that these disruptions were globules, vacuoles, multilamellar membranes and clusters of highly undulating membranes. Other potential scattering centers found throughout the mature cataract nucleus included variations in staining density between adjacent cells, enlarged extracellular spaces between undulating membrane pairs and protein-like deposits in the extracellular space. Subtle structural changes, especially small fluctuations in protein density between adjacent cells and alterations of the membranes and the extracellular space, probably contribute significantly to the central opacities in human nuclear cataracts (Costello et al., 1992). Such observations, considered as a whole, suggest that an understanding of the triggering mechanism of the lens fiber membrane damage is important to elucidate the causes of the lens ageing and human cataract formation. These manifestations are typical for the development of free-radical oxidation reactions in biological membranes (Babizhayev et al., 1988).
Kinetic curves of the accumulation of different molecule LPO products in the lens during cataract development correspond to the scheme of their gradual transformation in biological membranes when their autooxidation is being induced: phospholipids--- hydroperoxides of phospholipids---- carbonile compounds (MDA)---- intermolecular cross-links of Schiff bases type. This sequence of formation of lens phospholipid autooxidation products in the course of cataract development, provides a demonstration of the lenticular membrane LPO activation being a leading factor in cataractogenesis. The variety of the LPO products, as well as of the processes developing as a result of induction of autooxidation in lens fiber cells plasma membranes determines a large range of peroxidative effects on the opacification of the lens. The most characteristic manifestations can be included in these effects: disintegration of lenticular fiber plasma membranes, formation of new blue fluorophores, formation of high-molecular aggregates leading to light-diffusion in lenticular matter. Oxidation of membrane lipids could directly or indirectly alter the molecular structure of lens membranes. Development of peroxidative reaction in lipid membrane phase is accompanied by the accumulation of fluorescent products which have fluorescence excitation and emission spectra parameters similar to those of cataractous fluorophores (Babizhayev, 1989a, c). In our previous study a tentative investigation of physico-chemical nature of the fluorophores accumulated in lenticular lipids during cataract development, was undertaken (Babizhayev, 1989c). Protein oxidation in the lens has been shown to initiate at the membrane (Garner and Spector, 1980) and products of lipid peroxidation in the human lens increase with both age and cataract (Babizhayev, 1989a, c). In experimental animal models, LPO products have been reported to be a cause of cataract (Babizhayev and Deyev, 1989; Zigler et al., 1983; Horikawa et al., 1990). Borchman et al. (1989). has found that lipid oxidation is an early event in UVB-induced damage in lens epithelial membranes (Hightower et al., 1994). Together with polymerization of membrane-linked crystallins, the accumulation of LPO products in lenticular membranes can lead to an inhibition of membrane-bound enzymes, an impairment of protein-lipid interactions in membrane and also of molecular interactions of the membrane with cytoskeleton and water-soluble lenticular crystallins. LPO products can act as ion transfer inductors in the lens, increasing at corresponding stages of cataract development Na+ and Ca2+ ions intracellular concentration enhancing the opacification progress (Bhuyan et al., 1982-1983).
Which are the mechanisms of intermolecular protein cross-links formation in cataract? Dialdehyde-bifunctional reagents are characteristic of LPO products and their interaction with free aminogroups entails Schiff base forming and, subsequently, inter- and intramolecular cross-links. It should be pointed out that formation of phospholipid intermolecular cross-links has been registered in this study by characteristic fluorescence of lipid extract from cataractous lens. Covalent cross-linking of lenticular proteins in cataract may be possible also as a result of their interaction with the lipid-free radicals appearing in the course of LPO process. Cross-linking of biomolecules by the reaction of the carbonyl groups of MDA and amino groups of amino acids, proteins, nucleic acids and their bases and phospholipids produces lipofuscins, fluorescent Schiff-base conjugates resulting in formation of high molecular weight aggregates chronically accumulated in cataracts and increased in ripe cataracts as well as inactivating enzymes in the lens (Bhuyan and Bhuyan, 1984; Babizhayev et al., 1988; Babizhayev, 1989a, c).
The primary molecular LPO products, i.e., phospholipid hydroperoxides and their in vivo indicator, diene conjugate, represent the major change in the lipid composition of aqueous humor during cataract formation. At the stages of ripe cataract, end fluorescent LPO products are distinctly detected. Crystalline lens is well equipped with antioxidant defences showing some specificity in their action. LPO in the lens incubation system was decreased in the presence of free radical scavengers and enzymes that degrade H2O2 (EDTA, SOD, catalase, L-carnosine and chelated iron). The most effective agent was EDTA which chelates the free metal cations required to generate O2¯• radicals that initiate the free radical process culminating in LPO. The mechanism of metal-catalysed oxidation reactions of the lens reductants (GSH, ascorbate, lens crystallins containing SH groups) was proposed as a basic for release of free radicals or lipid hydroperoxides in the lens medium and the rates and final levels of oxidants formation by lenses were found inversely dependent to their integral degrees of clouding (Babizhayev and Bozzo Costa, 1994). Since LPO is clinically important in many of the pathological effects and ageing, new therapeutic modalities should treat the incessant infliction of damage to the lens cells and biomolecules by reactive lipid peroxides and oxygen species and ‘refashion’ the affected lens membranes in the lack of important metabolic ‘detoxification’ of phospholipid peroxides. L-Carnosine and its ophthalmic prodrug form N-acetylcarnosine are part of this group of products (Babizhayev, 1996; Babizhayev et al., 1994; 1996 ). These compounds act as universal antioxidants with established ability to give efficient protection against LPO both in the lipid phase of cellular membranes and in aqueous environment (protecting proteins, DNA, sugars from oxidative damage). Various protective antioxidant enzymes such as SOD or catalase can only react with their substrates in aqueous environment (Halliwell and Gutteridge, 1985). The data of this study demonstrate that when a naturally occurring compound N- acetyl-carnosine is combined in the ophthalmic formulation Can-CTM with a lubricant carboxymethylcellulose, an increased uptake of L-carnosine in the aqueous humor occurs in the topically treated rabbit eyes over the application of pure N-acetylcarnosine compound in the control buffered ophthalmic formulations.
The ophthalmic topical application of pure L-carnosine (1% solution) to the rabbit eyes (instillation, subconjunctival injection, ultrasound-induced phono-phoresis) to produce the desired effect, whether this is therapeutic or prophylactic, did not lead to the intraocular absorption or accumulation in the aqueous humor of this dipeptide compound (Babizhayev et al., 1996). Thus the pharmaceutical compounding and formulating of N-acetylcarnosine drug into suitable dosage forms for human and veterinary administration is important according to the developed pharmaceutical formulations (Babizhayev, 2004) describing a combination of a pharmacologically active N-acetylcarnosine with a cellulose compound to enhance activity, reduce side effects of pure L-carnosine and to modify the drug’s action in a way to make it more suitable for a treatment of cataracts.
Additionally, after absorption in the aqueous humor from the ophthalmic time release N-acetylcarnosine carrier, L-carnosine must pass into the lens according to provided studies gaining access to prevent and manage the oxidative damage to the lens cells and tissues. In the present study we have in vitro assessed the uptake of L-carnosine in the crystalline lens as antioxidant at the viable contents produced during the chronical usage of the pharmaceutical drug form required for the patient to self-medicate (Babizhayev, 2004; Babizhayev et al., 2004). The visualized effects of 1% N-acetylcarnosine with lubricant carboxymethylcellulose on age-related cataracts in the clinical cases were also demonstrated by the author’s group (Seidler et al., 2004). These observations suggest that N-acetylcarnosine may prevent cataracts.
However, there is also evidence suggesting that N-acetylcarnosine reverses lens opacity in humans (Babizhayev et al., 2002; 2001; 2000a). The mechanism for this observation may involve carnosine’s ability to disaggregate glycated α-crystallin protein (Seidler et al., 2004). In the cataractous lens, cross-linking of proteins by any means increases their effective molecular weight and produces light scattering and consequent lenticular opacity. The production of such high molecular weight protein complexes by disulphide bridges and covalent links with dialdehydes has been implicated in the formation of senile and other cataracts (Babizhayev and Deyev, 1986). Both types of cross-linking may be caused by depletion of the lens’ reduced glutathione and accumulation of LPO products in the lens tissue. The results of our studies strongly suggest that L-carnosine released from its ophthalmic prodrug N-acetylcarnosine during its topical application to the eyes with cataracts is able to prevent the loss of reduced glutathione and to remove the secondary LPO products in biological systems (Babizhayev and Deyev, 1989). This, in turn, may lead to dissociation of the intermolecular protein cross-links due to glutathione-protein thiol-disulphide exchange mechanism and utilization of lipid peroxides and dialdehydes derived from LPO process, anchoring protein-lipid complexes in the lens (Babizhayev and Deyev, 1989). A possibility exists from our studies that carnosine is reacting directly with MDA and other aldehydes/ketones. Indeed carnosine has been shown to protect against MDA-induced crosslinking and toxicity and a hydroxynonenal-carnosine adduct has recently been characterized, providing further evidence for carnosine’s potential as an aldehyde scavenger (Aldini et al., 2002). The presented results can be explained in part by the adduction of the various LPO products directly by carnosine following de-acetylation of N-acetylcarnosine. The published results suggest that histidine is the representative structure of L-carnosine for an anti-crosslinking agent, containing the necessary functional groups for optimal protection against crosslinking agents (Hobart et al., 2004).
The normal metabolic processes are essential for cell growth replacement and, in the case of the corneal epithelium and endothelium, for themaintenance of the ionic pump mechanism, which is responsible for the maintenance of the state of corneal hydration. In this study we evaluated the hypothesis that contact-lens-induced stromal acidosis causes corneal complications. A goal of the present study was to develop a practical clinical method or process to compensate stromal pH so that these research findings may have clinical applications. We are currently in the process of analyzing the completed clinical database. Intracellular pH (pH(i)) is an important modulator of the corneal function. Because it is readily influenced by metabolic processes, pH(i) is controlled physiologically. The data support the clinical application of developed N-acetylcarnosine lubricant eye drops to compensate corneal acidosis during contact lens wear which is significant under a variety of wearing conditions and, thus, must be considered when evaluating the morphological or functional changes that occur following long-term contact lens wear. Intracellular H(+) corneal buffering is adjusted and occurs through a shuttling of the intrinsic mobile buffers such as acetylated carnosine and its bioactivating ophthalmic ingredient carnosine low molecular weight imidazole compounds.
LPO reactions are widely involved in the genesis of ophthalmic disorders, such as cataract, glaucoma, inflammatory, corneal, retinal, systemic disorders having a component of oxidative stress in their genesis (Babizhayev, 1996). These are the most widely used application sites for drugs and pharmaceutical products besides ophthalmic surgery. But mainly products are swallowed in order that the active ingredient can be absorbed from the gut and gain access to the blood stream and in this way, secondary to the eye. While some parts of the eye are richly supplied with blood vessels, others including the crystalline lens are not. Moreover, the response of systemic drugs and oral supplements can be variable as other gut contents can influence the absorption of alimentary-administered remedies and particularly, being mostly peptidergic in nature products, are susceptible to preoteolytic breakdown by proteases, dipeptidases, encountered during internal digestion in the gastro-intestinal tract or transport in the blood stream and consequently have too limited half- life upon systemic application. The present study presents a pharmacokinetic evidence suggesting that the developed ophthalmic prodrug N-acetylcarnosine time-release form (Can-CTM) is most effective to make L-carnosine bioavailable for the intraocular absoption in the aqueous humor. This is by far the most effective route of administration of L-carnosine for the eye and forwarding it to the lens tissues. Topically applied ophthalmic formulation produces effective levels of L-carnosine in the anterior segment of the eye. The response of the patient to the drug will to a great extend depend on the concentration of L-carnosine that is available at the site of action near the lens in the aqueous humor. The relationship between the dose or concentration administered and the final L-carnosine concentration at the focus of action near the lens in the anterior segment of the eye is the resultant of normal pharmacokinetic processes of N-acetylcarnosine deacetylation which are themselves subject to the effect of other agents and excipients (such as carboxymethylcellulose, vitamins, buffers, preservatives, pH adjusters) (Babizhayev, 2004). Present studies document the optimal bioavailability of an ophthalmic formulation including 1% N-acetylcarnosine with a lubricant carboxymethylcellulose and predict further benefits of the application of this drug in prevention and reversal of age-related cataracts in human and in canine eyes (Babizhayev, 2004; Babizhayev et al., 2004). Other than senile cataract, N-acetylcarnosine may have other clinical benefits. The unique and patented N-acetylcarnosine lubricant eye drops formula could also provide beneficial results with the following eye-disorders (Babizhayev, 2004):
| • | Presbyopia. |
| • | Open-angle primary glaucoma (in combination with beta-blockers). |
| • | Corneal disorders |
| • | Computer vision syndrome. |
| • | Eyestrain |
| • | Ocular inflammation. |
| • | Blurred vision. |
| • | Dry eye syndrome. |
| • | Retinal diseases. |
| • | Vitreous opacities and lesions. |
| • | Complications of diabetes mellitus and other systemic diseases. |
| • | Contact lens difficulties, particularly with soft contact lenses. (Not only do the lubricants in the Can-C N-acetylcarnosine eye-drop help to make wearing contact lenses more comfortable, but n-acetylcarnosine is also believed to reduce the build up of lactic acid in the eye, thus enabling the lens to be left safely in the eye for longer). |
N-acetylcarnosine applied topically to the eye and moreover, its time released ophthalmic ingredient L-carnosine exerts anti-glycation, bioactivating antioxidant properties in the lens and cornea as a scavenger of lipid peroxides, singlet oxygen and OH• radicals and spatial aspects of intracellular pH regulation (Babizhayev et al., 2006).
This study was planned, organized and supported by Innovative Vision Products, Inc., at address of 3511 Silverside Road, Suite 105, County of New Castle, Delaware, USA 19810. Innovative Vision Products, Inc. is a holder of the worldwide patent (including PCT International Publication Number WO 2004/028536 A1) for the application of N-acetylcarnosine for the treatment of ophthalmic disorders including cataracts.