Research Article
Phylogenetic Analysis and Protein Modeling of Plasmodium falciparum Aspartate Transcarbamoylase (ATCase)
Microbiology and Biotechnology Laboratory, Faculty of Animal Sciences, Mataram University, Jl. Majapahit No. 62, Mataram, NTB, 83125, Indonesia
I. Menz
School of Biological Sciences, Flinders University of South Australia, GPO Box 2100, Adelaide 5001, South Australia









florence mactouff Reply
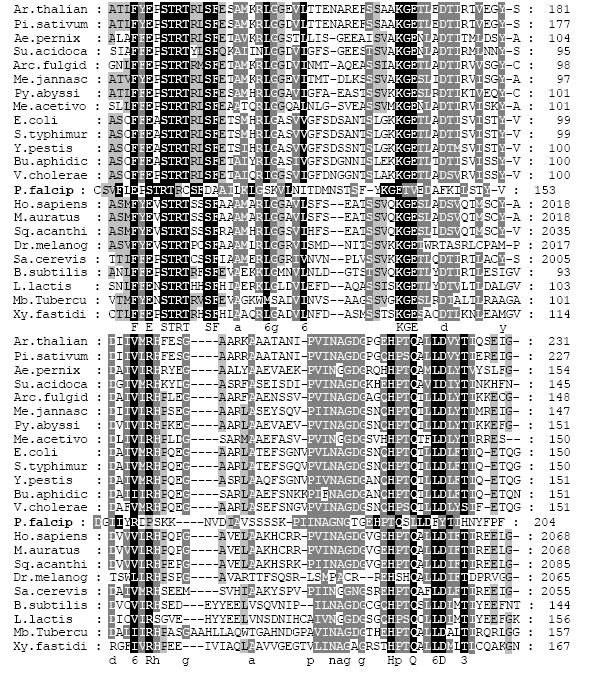
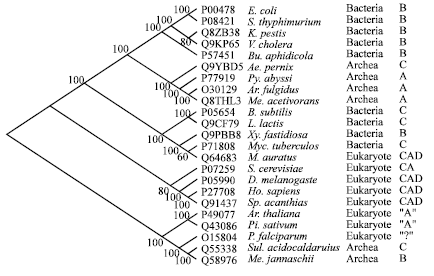
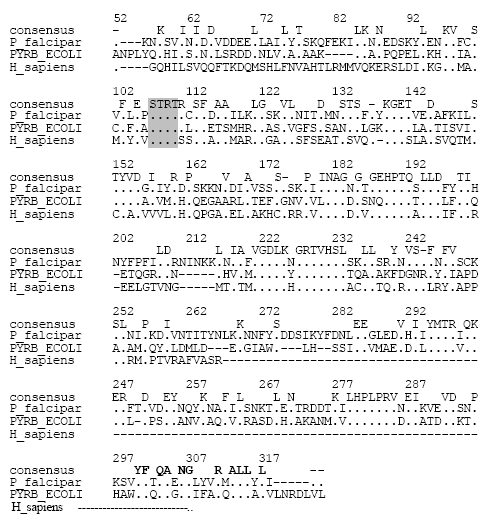
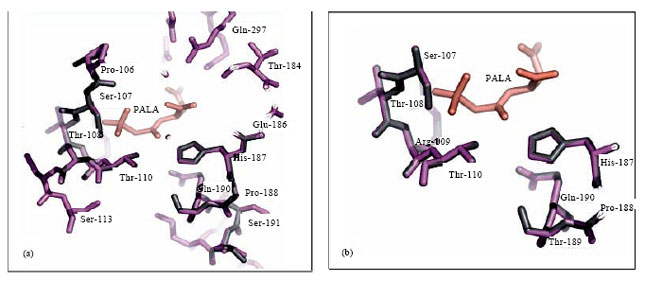
What are the major structural differences between Plasmodium falciparum ATCASE and human host enzyme.