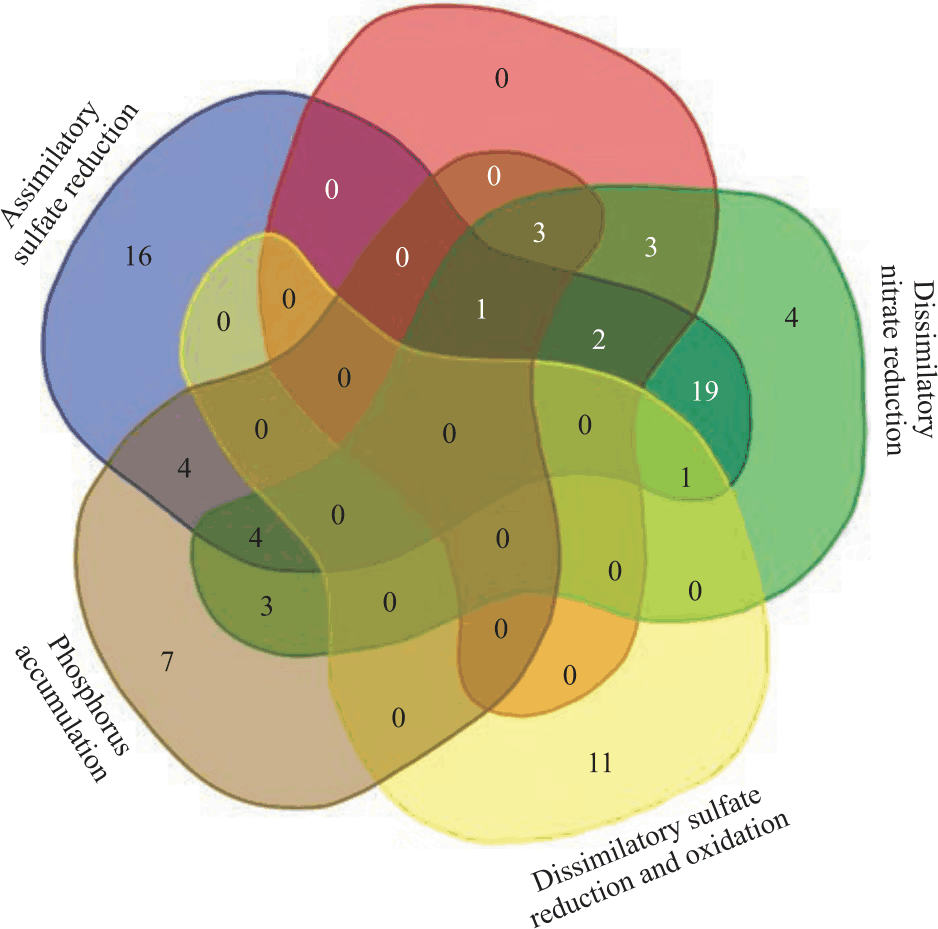
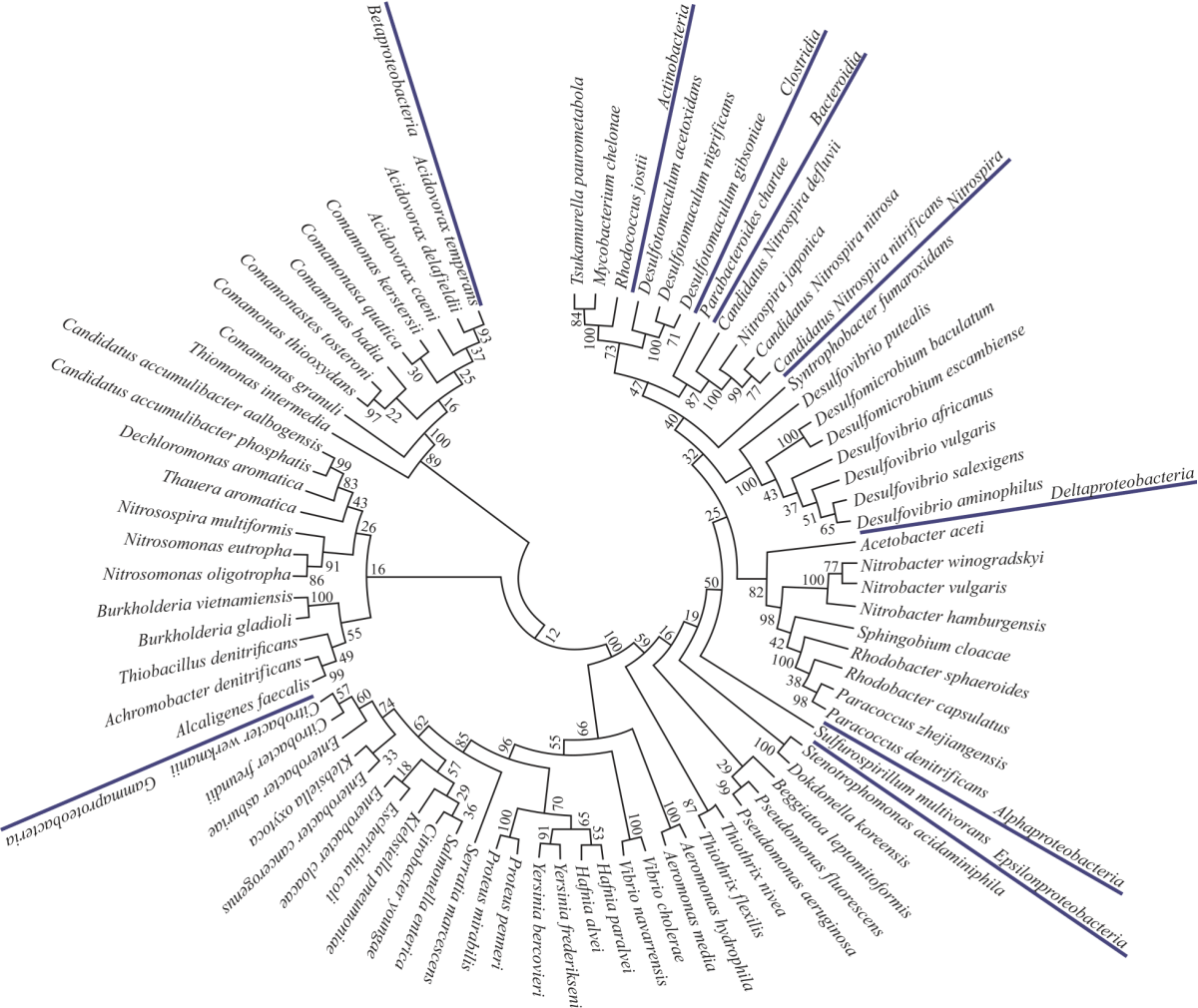
Research Article
Identification of Bacterial Species with Nitrogen, Phosphorus and Sulfur Bioremediation Pathways in Wastewater Treatment Plants
Instituto de Biotecnologia, Universidade Federal de Uberlândia, Patos de Minas, Minas Gerais, Brazil
Aulus Estevão Anjos de Deus Barbosa
Instituto de Biotecnologia, Universidade Federal de Uberlândia, Patos de Minas, Minas Gerais, Brazil
LiveDNA: 55.32664