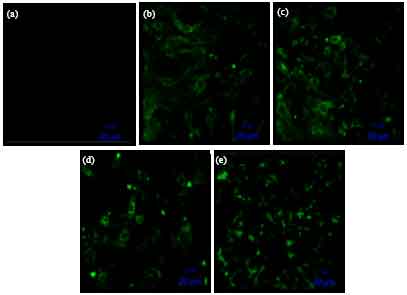
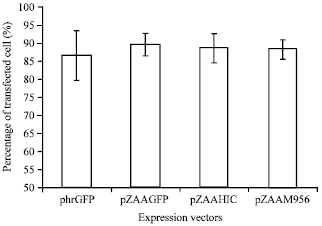
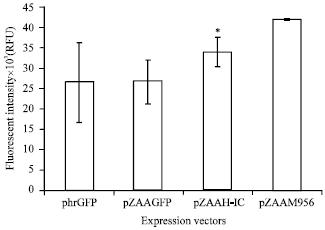
Short Communication
Identification of Transfection Efficiency Using Qualitative and Quantitative Analyses of Green Fluorescent Protein in CHO Cells
School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Z. Zulkiflie
Inno Biologics Sdn Bhd, Lot 1 Persiaran Negeri BBN, Putra Nilai 71800, Nilai, Negeri Sembilan, Malaysia
M.A.W. Rohaya
Department of Orthodontics, Faculty of Dentistry, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, 50300 Kuala Lumpur, Malaysia
S. Sahidan
School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
Z.A. Intan Zarina
School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
M.Y. Nurul Yuziana
School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia
M. Zulkifli
Department of Neuroscience, School of Medical Sciences, Universiti Sains Malaysia, Health Campus, 16150, Kubang Kerian, Kelantan, Malaysia
Z.A. Shahrul Hisham
School of Biosciences and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia