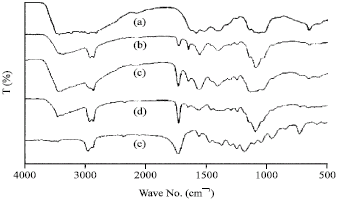
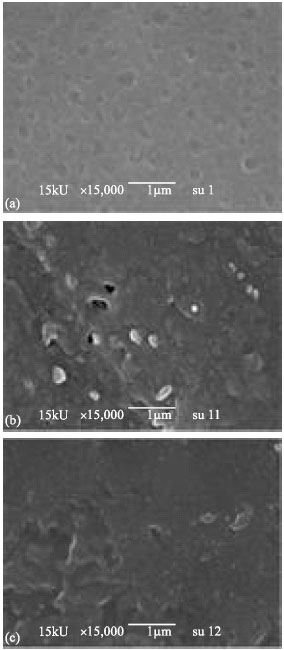
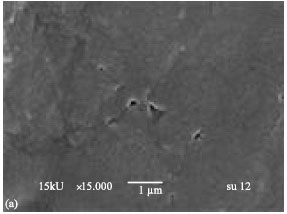
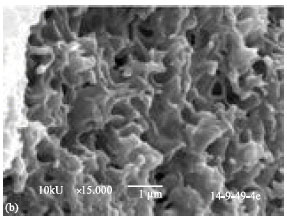
Research Article
Morphology and Thermal Stability of Chitosan and Methoxy Poly (ethylene glycol)-b-Poly (ε-caprolactone)/Poly(D, L-lactide) Nanocomposite Films
Center of Excellence for Innovation in Chemistry, Department of Chemistry Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand
Yodthong Baimark
Center of Excellence for Innovation in Chemistry, Department of Chemistry Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand