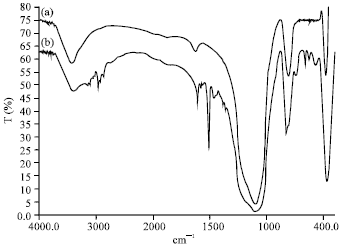
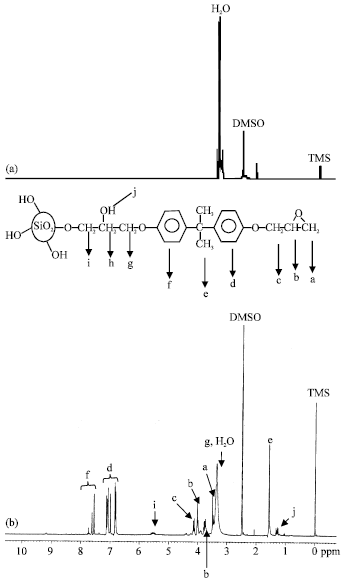
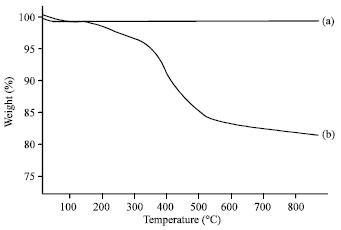
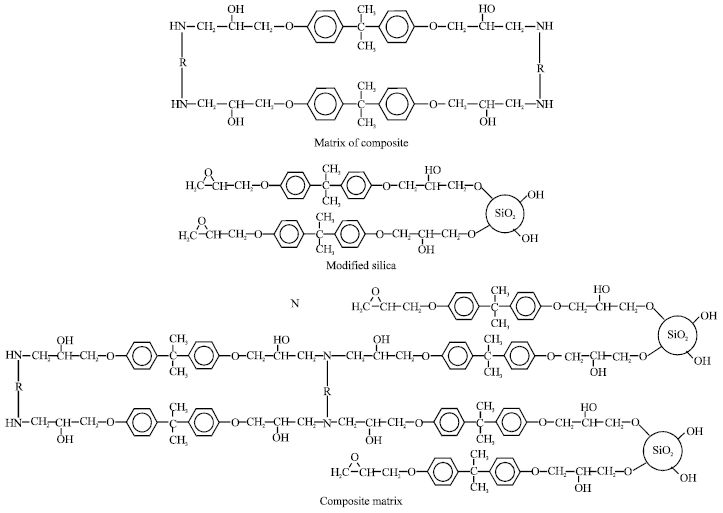
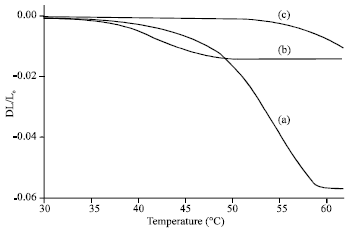
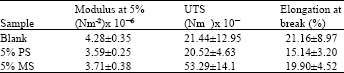
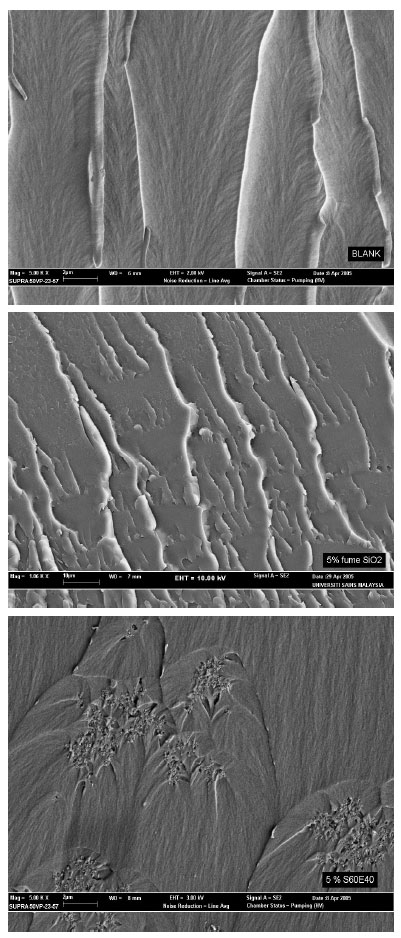
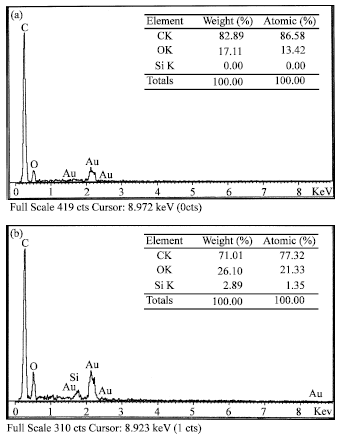
Research Article
Properties and Morphology of Bulk Epoxy Composites Filled with Modified Fumed Silica-Epoxy Nanocomposites
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
N. Ahmad
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
R. Adnan
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
I.Ab. Rahman
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
M.A. Bakar
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
J. Ismail
School of Chemical Sciences, Universiti Sains Malaysia, 1 1 800 P. Pinang, Malaysia
C.K. Chee
1nte1 Technology (M) Sdn. Bhd, Bayan Lepas FIZ Phase 111, 1 1900 P. Pinang, Malaysia