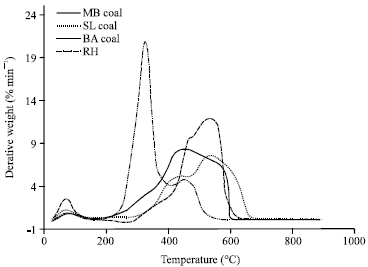
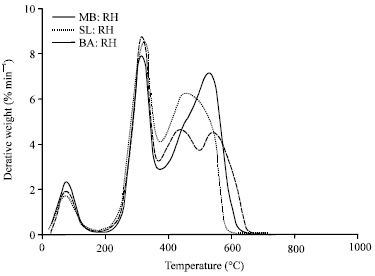
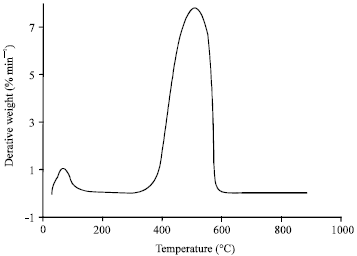
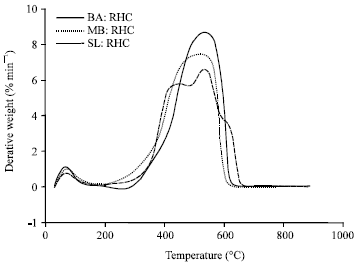
Research Article
Thermal Decomposition Study of Coals, Rice Husk, Rice Husk Char and Their Blends During Pyrolysis and Combustion via Thermogravimetric Analysis
Faculty of Applied Sciences, University Technology MARA Malaysia, 40000 Shah Alam, Selangor, Malaysia
M.A. Mohd Ishak
Faculty of Applied Sciences, University Technology MARA Malaysia, 40000 Shah Alam, Selangor, Malaysia
M.F. Abdullah
Faculty of Applied Sciences, University Technology MARA Malaysia, 40000 Shah Alam, Selangor, Malaysia
K. Ismail
Faculty of Applied Sciences, University Technology MARA Malaysia, 40000 Shah Alam, Selangor, Malaysia