Research Article
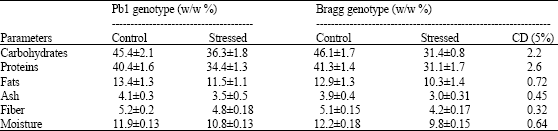
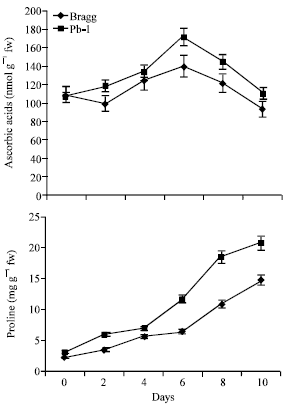
Water-Deficit Stress During Seed Filling in Contrasting Soybean Genotypes: Association of Stress Sensitivity with Profiles of Osmolytes and Antioxidants
Department of Botany, Panjab University, Chandigarh 160 014, India
S. Kaur
Department of Botany, Panjab University, Chandigarh 160 014, India
K. Singh
Department of Botany, Panjab University, Chandigarh 160 014, India
D. Pathania
Department of Botany, Panjab University, Chandigarh 160 014, India
N. Kaur
Department of Botany, Panjab University, Chandigarh 160 014, India
S. Sharma
Department of Botany, Panjab University, Chandigarh 160 014, India
H. Nayyar
Department of Botany, Panjab University, Chandigarh 160 014, India









Frank sinota Reply
nice study. can be extended to differential gene display using these cultivars to corroborate the expression of these molecules